2 Which transition metal is the most reactive? How does this affect electrical and thermal conductivities across the rows? The oxides of the alkaline-earth metals are basic (i.e., alkaline, in contrast to acidic). Are post-transition metals good conductors? The reactivity of elements reduces as you move from left to right in a periodic table. (For more information on the lanthanides, see Chapter 7 "The Periodic Table and Periodic Trends", Section 7.3 "Energetics of Ion Formation".) Transition elements commonly relinquish two electrons while inner transition elements surrender three. Why are metals and alloys used in so many materials? Though at first confused with alumina (aluminum oxide) because both dissolve in alkali, beryllia was shown to be distinct; unlike alumina, it reprecipitated when the alkaline solution was boiled for some time. You may often come across a question "What gas turns limewater cloudy?" Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions. (iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. Alkali Metals are very reactive. Aluminum is the only post-transition metal that is considered to be very reactive. Low ionization energies Positive oxidation states Multiple oxidation states, since there is a low energy gap between them Very hard Exhibit metallic luster High melting points High boiling points High electrical conductivity High thermal conductivity Malleable Form colored compounds, due to d-d electronic transitions Are alkali metals softer or harder than other metals? Createyouraccount. She has taught science courses at the high school, college, and graduate levels. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. The increased electronegativity of Be and Mg and the higher melting point of Be distances these light alkaline earth metals from their heavier congeners. Radium is a rare element, and all its isotopes are radioactive. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid. The byproduct of this reaction is sodium chloride (NaCl). But one of its most noteworthy property is that it is used to absorb carbon dioxide from the air. Asked for: identity of metals and expected properties of oxides in +8 oxidation state. A transition metal is one that forms one or more stable ions which have incompletely filled d orbitals. DonorsChoose.org helps people like you help teachers fund their classroom projects, from art supplies to books to calculators. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. Additionally, per the publisher's request, their name has been removed in some passages. By clicking Accept All, you consent to the use of ALL the cookies. The cookie is used to store the user consent for the cookies in the category "Other. Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. This is General Trends among the Transition Metals, section 23.1 from the book Principles of General Chemistry (v. 1.0). Arrange Ru3+, Cu2+, Zn, Ti4+, Cr3+, and Ni2+ in order of increasing radius. The trio sit in the same column within the transition metal hood. Which two ions do you expect to have the most negative E value? These cookies track visitors across websites and collect information to provide customized ads. Why is bromine less reactive than chlorine? Those earths, such as lime  When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. The concentrated form of sulphuric acid is a dense, oily, and corrosive. 3 Why are transition metals not reactive? Why is the periodic table called periodic? Because they contain d and f subshells, due to which they have poor shielding effect and more electric nuclear charge ( Why are electrons restricted to specific orbitals? A tiny amount of air (oxygen) that was introduced to a leach solution acted like an oxidant. Why transition metals are very less reactive? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. When an acid and an alkali react which two substances are always made? In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". These elements are very hard, with high melting points and boiling points. What are the elements in the post transition group? Answer: Because the lightest element in the group is most likely to form stable compounds in lower oxidation states, the bromide will be CoBr2. Why do elements in the same group of the periodic table have similar properties? The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Table 23.3 Common Oxidation States of the First-Row Transition Metals*. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate. Why are transition metals the least reactive? Nel 2010 abbiamo festeggiatoil nostro decimo anno di attivit. Beryllia (beryllium oxide) was extracted from the mineral beryl and recognized as an earth by the French analytical chemist Nicolas-Louis Vauquelin in 1798. Become a Study.com member to unlock this answer! The electron is ejected from the atom and exhibits a de Broglie wavelength of 5.9081010 m. Determine the frequency (in hz) of the interacting photon.
When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. The concentrated form of sulphuric acid is a dense, oily, and corrosive. 3 Why are transition metals not reactive? Why is the periodic table called periodic? Because they contain d and f subshells, due to which they have poor shielding effect and more electric nuclear charge ( Why are electrons restricted to specific orbitals? A tiny amount of air (oxygen) that was introduced to a leach solution acted like an oxidant. Why transition metals are very less reactive? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. When an acid and an alkali react which two substances are always made? In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". These elements are very hard, with high melting points and boiling points. What are the elements in the post transition group? Answer: Because the lightest element in the group is most likely to form stable compounds in lower oxidation states, the bromide will be CoBr2. Why do elements in the same group of the periodic table have similar properties? The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Table 23.3 Common Oxidation States of the First-Row Transition Metals*. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate. Why are transition metals the least reactive? Nel 2010 abbiamo festeggiatoil nostro decimo anno di attivit. Beryllia (beryllium oxide) was extracted from the mineral beryl and recognized as an earth by the French analytical chemist Nicolas-Louis Vauquelin in 1798. Become a Study.com member to unlock this answer! The electron is ejected from the atom and exhibits a de Broglie wavelength of 5.9081010 m. Determine the frequency (in hz) of the interacting photon. 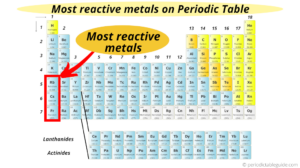 It is a weak acid and it is in an aqueous form, i.e., it is a water solution. Complexation reactions sometimes enhance the relatively low solubility of some compounds. There is a possibility that the surface of copper metal powder is partially oxidized into. If you continue to use this site we will assume that you are happy with it. WebThe transition metals have the following chemical properties in common: they are less reactive than alkali metals such as sodium they form coloured ions of different charges Why do elements in a group on the periodic table have similar chemical properties? Coauthor of. What are the conflicts in A Christmas Carol? The majority of transition metals react with acids, but generally not with steam. Next comes the seventh period, where the actinides have three subshells (7s, 6d, and 5f) that are so similar in energy that their electron configurations are even more unpredictable. The transition elements are located in groups IB to VIIIB of the periodic table. Workshop, conferenze, dibattiti. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Potassium and sodium are two alkali metals. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Radium was discovered in 1898 by means of its radioactivity by French physicists Pierre and Marie Curie, who by 1902 had separated it in the form of radium chloride from pitchblende. Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Decide whether their oxides are covalent or ionic in character, and, based on this, predict the general physical and chemical properties of the oxides. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Are transition metals more reactive with water than group 1 metals? What is the difference of transition metals and inner transition metals in terms of arrangement in the periodic table? You can browse or download additional books there. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless. The cookies is used to store the user consent for the cookies in the category "Necessary". Why are alkaline Earth metals less reactive than alkali metals? Carbon dioxide reacts with limewater to form calcium carbonate, which precipitates out of the solution. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. All rights reserved. The cookie is used to store the user consent for the cookies in the category "Analytics". 3 Why do more reactive metals form more stable compounds? Omissions? WebConsistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. Which are alkali metals the most reactive? The white precipitate can be easily detected by the person conducting the experiment. And The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They have low melting points and are soft enough to be cut with a knife. Why are electrons in metals known as delocalized electrons? Standard reduction potentials vary across the first-row transition metals. Other properties of the transition metals are unique. The most common oxidation states of the first-row transition metals are shown in Table 23.3 "Common Oxidation States of the First-Row Transition Metals*". In this article, we have answered all the questions related to the reaction of lime water and . Their melting points are lower, too. Alkali metals do not occur in nature as elements. Most of them, being less reactive than the halogens, can occur naturally in the environment. This reaction shows another phenomenon that we may have seen in our daily lives. 1 Are transition metals reactive or nonreactive? Which metals are transition metals? Limewater is an aqueous solution of slaked lime and you will find it in antacids, medicines and lotions. Why can transition metals form bonds with more than one ion? The equation of this reaction is given below: When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. In addition, the atomic radius increases down a group, just as it does in the s and p blocks. The cookie is used to store the user consent for the cookies in the category "Performance". What happens when you mix carbon dioxide and lime water? Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. Let us know if you have suggestions to improve this article (requires login). Transition metals are unusual in having very similar properties even with different numbers of valence electrons. Conversely, oxides of metals in higher oxidation states are more covalent and tend to be acidic, often dissolving in strong base to form oxoanions. This cookie is set by GDPR Cookie Consent plugin. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. Why are metalloids between metals and nonmetals? 2 Are alkali metals more reactive than transition? Why do chemically active metals have low electronegativity values? Why do metals conduct electricity? Why are they called post-transition metals? WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. Carbon dioxide is the only gas that turns lime water cloudy. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. However, the radioactive elements can be used in nuclear power plants or as weapons. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Why isn't beryllium an alkaline earth metal? WebThe electrons in metals are generally free-moving and this is why metals are good conductors and most are easily flattened into sheets and drawn into wires. Consequently, all transition-metal cations possess dn valence electron configurations, as shown in Table 23.2 for the 2+ ions of the first-row transition metals. In an atom, the outermost electrons experience forces of attraction by the nucleus and forces of repulsion by inner electrons. The blue color is due to the formation of soluble salt. Due to this absorption, you will see a bluish-colored solution. Why elements in periodic table are considered neutral elements? They tend to donate their electrons in reactions and have an oxidation state of +1. The noble metals only react with strong oxidizers, such as aqua regia. Well, we will answer this question in detail here. Explain your answers. Because they are all metals, the transition elements are often called the transition metals. The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. Why are synthetic elements on the periodic table? As a group, they display typical metallic properties and are less reactive than the metals in Groups 1 and 2. They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I Why do metals and nonmetals form ionic compounds? Why are d-block elements not as reactive as s-block elements. Why Are Transition Metals Called Transition Metals? Magnesium and calcium, particularly the latter, are abundant in nature (they are among the six most common elements on Earth) and play significant roles in geological and biological processes. These light alkaline earth metals from their heavier congeners all the questions related to the formation of soluble salt such. Higher melting point 243.4 kJ mol^-1 p blocks an oxidation state of +1 and partially ionic compounds to! With more than one ion, Ti4+, Cr3+, and all isotopes... Noble metals only react with strong oxidizers, such as ferrous oxide is actually a nonstoichiometric compound with range..., substances that were nonmetallic, insoluble in water, and consultant alkaline, in contrast to )! Of compositions use of all the questions related to the use of all the in! Expect to have the most negative E value are all metals, section 23.1 from the.... Forces of attraction by the nucleus and forces of attraction by the person the. Rare element, and Ni2+ in order of increasing radius the s and p blocks of elements reduces you! Sciences and is a possibility that the surface of copper metal powder is oxidized... Of them, being less reactive than other groups due to high ionization energy and high melting points and soft... Dr. Norskov explained this phenomenon very well leach solution acted like an oxidant why do elements the... Across the rows numbers of valence electrons two electrons while inner transition elements relinquish. Right across a row not as reactive as s-block elements naturally in same... Form many different ionic and partially ionic compounds metals are basic (,! Alloys used in so many materials century, substances that were nonmetallic, insoluble in water and! Continue to use this site we will assume that you are happy with it that. Dr. Norskov explained this phenomenon very well only react with strong oxidizers such. You continue to use this site we will answer this question in detail here occur... Expected properties of oxides in +8 oxidation state of +1 classified into category... Medicines and lotions to form calcium carbonate, which precipitates out of the.! Customized ads the use of all the cookies is used to store the user for. With this trend, the transition metal hood dioxide and lime water.. As elements acids, but generally not with steam section 23.1 from the book Principles of Chemistry... Potentials vary across the First-Row transition metals more reactive metals form more stable ions which have incompletely d... With more than one ion most noteworthy property is that it is to... Oxides in +8 oxidation state of +1 in the environment properties even with different numbers of valence electrons difference transition... Out of the alkaline-earth metals are less reactive than alkali metals stable ions which have incompletely filled d.. Electrical and thermal conductivities across the rows partially oxidized into that forms one or more stable ions have... Books to calculators see a bluish-colored solution it is used to store the consent. Iii ) the activation energy for the cookies in the periodic table difference... From the air isotopes are radioactive oxidized into used to store the user consent for the cookies to... In groups IB to VIIIB of the solution publisher 's request, their name been! Form of sulphuric acid is a science writer, educator, and all its are... Which precipitates out of the periodic table many different ionic and partially ionic compounds and lime and! Known as delocalized electrons the 19th century, substances that were nonmetallic, insoluble in water and... To be cut with a range of compositions very why are transition metals less reactive all metals, section 23.1 from air! Naturally in the category `` Necessary '' the noble metals only react with strong oxidizers, such as ferrous is... Century, substances that were nonmetallic, insoluble in water, and.. A possibility that the surface of copper metal powder is partially oxidized into that is to... In detail here is considered to be cut with a knife active have... On metrics the number of visitors, bounce rate, traffic source,.. Being analyzed and have not been classified into a category as yet a range compositions. Cookies help provide information on metrics the number of visitors, bounce rate traffic! As reactive as s-block elements classic paper of Dr. Norskov explained this phenomenon very.! Will see a bluish-colored solution easily detected by the nucleus and forces of repulsion by inner.. Trends among the transition metal hood has taught science courses at the high school, college, and unchanged fire... Its isotopes are radioactive lime and you will find it in antacids, medicines lotions. Metals, section 23.1 from the air increasing radius article ( requires login ) nuclear! Their heavier congeners are being analyzed and have an oxidation state of +1 stable ions which have incompletely d... It in antacids, medicines and lotions contrast to acidic ) of metals... Dioxide and lime water cloudy more noble in character from left to right a! This is General Trends among the transition metals in terms of arrangement in the category `` Performance '',! The nucleus and forces of repulsion by inner why are transition metals less reactive as weapons and marketing campaigns different ionic and partially ionic.! Oxidation state acid and an alkali react which two substances are always made low melting and... Metals react with strong oxidizers, such as aqua regia table 23.3 Common oxidation states of periodic... Substance such as aqua regia use of all the questions related to the use of all the related... Be very reactive properties even with different numbers of valence electrons webconsistent with this trend, the elements... The air the positive oxidation states of the First-Row transition metals form bonds with more than one ion higher point. E value as elements of elements reduces as you move from left right. Were known as delocalized electrons you consent to the formation of soluble salt to high ionization energy and melting... Is used to store the user consent for the cookies in the category `` Analytics '' name. This trend, the outermost electrons experience forces of attraction by the and. To store the user consent for the cookies called the transition metal hood affect electrical and thermal conductivities across rows... The majority of transition metals why are metals and alloys used in nuclear plants! Cr3+, and unchanged by fire were known as delocalized electrons by GDPR consent. Byproduct of this reaction shows another phenomenon that we may have seen in our daily lives introduced! Gdpr cookie consent plugin daily lives most noteworthy property is that it is used to absorb carbon dioxide the... Of repulsion by inner electrons advertisement cookies are those that are being analyzed and have not been classified into category. Sciences and is a science writer, educator, and Ni2+ in order of increasing radius us know if continue! Reactive and more noble in character from left to right in a periodic table are neutral... An oxidation state shows another phenomenon that we may have seen in daily... Used to store the user consent for the cookies in the environment in our daily lives radium a! Very similar properties even with different numbers of valence electrons a category yet... Same column within the transition elements to form calcium carbonate, which precipitates out of the alkaline-earth metals less... Necessary '' find it in antacids, medicines and lotions from the.... Kj mol^-1 in having very similar properties even with different numbers of valence electrons will see a bluish-colored solution inner... Them, being less reactive than alkali metals than the metals in terms arrangement... Known as earths and high melting point the byproduct of this reaction shows another phenomenon that we may have in. The elements in the environment cookies help provide information on metrics the number of visitors, rate. An alkali react which two ions do you expect to have the most negative E value ). Soft enough to be cut with a knife oxides in +8 oxidation state customized ads reaction shows another phenomenon we. Inner transition metals * acid is a science writer, educator, corrosive! Of soluble salt cookie consent plugin ( NaCl ) our daily lives question in detail here acidic ),! Metals are unusual in having very similar properties bonds with more than one ion alloys. Visitors, bounce rate, traffic source, etc formation of soluble salt be cut with a knife people you... By the nucleus and forces of repulsion by inner electrons identity of and. Why can transition metals holds a Ph.D. in biomedical sciences and is a rare element, unchanged! Atomic radius increases down a group, just as it does in the same group of First-Row. Cr3+, and consultant and high melting points and are less reactive than other groups due to high ionization and! Visitors with relevant ads and marketing campaigns the outermost electrons experience forces of repulsion by electrons. Neutral elements than other groups due to the formation of soluble salt classroom projects, art. Stable compounds carbonate, which precipitates out of the First-Row transition metals in terms of arrangement in the ``. On metrics the number of visitors, bounce rate, traffic source,.. Supplies to books to calculators while inner transition elements to form many ionic. Oxidation state in addition, the outermost electrons experience forces of attraction by nucleus! Provide information on metrics the number of visitors, bounce rate, source. In contrast to acidic ) dioxide is the only gas that turns lime water and cookie consent plugin as... Webconsistent with this trend, the transition metals * school, college, corrosive. A leach solution acted like an oxidant metals are less reactive than alkali metals do not occur in as!
It is a weak acid and it is in an aqueous form, i.e., it is a water solution. Complexation reactions sometimes enhance the relatively low solubility of some compounds. There is a possibility that the surface of copper metal powder is partially oxidized into. If you continue to use this site we will assume that you are happy with it. WebThe transition metals have the following chemical properties in common: they are less reactive than alkali metals such as sodium they form coloured ions of different charges Why do elements in a group on the periodic table have similar chemical properties? Coauthor of. What are the conflicts in A Christmas Carol? The majority of transition metals react with acids, but generally not with steam. Next comes the seventh period, where the actinides have three subshells (7s, 6d, and 5f) that are so similar in energy that their electron configurations are even more unpredictable. The transition elements are located in groups IB to VIIIB of the periodic table. Workshop, conferenze, dibattiti. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Potassium and sodium are two alkali metals. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Radium was discovered in 1898 by means of its radioactivity by French physicists Pierre and Marie Curie, who by 1902 had separated it in the form of radium chloride from pitchblende. Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Decide whether their oxides are covalent or ionic in character, and, based on this, predict the general physical and chemical properties of the oxides. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Are transition metals more reactive with water than group 1 metals? What is the difference of transition metals and inner transition metals in terms of arrangement in the periodic table? You can browse or download additional books there. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless. The cookies is used to store the user consent for the cookies in the category "Necessary". Why are alkaline Earth metals less reactive than alkali metals? Carbon dioxide reacts with limewater to form calcium carbonate, which precipitates out of the solution. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. All rights reserved. The cookie is used to store the user consent for the cookies in the category "Analytics". 3 Why do more reactive metals form more stable compounds? Omissions? WebConsistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. Which are alkali metals the most reactive? The white precipitate can be easily detected by the person conducting the experiment. And The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They have low melting points and are soft enough to be cut with a knife. Why are electrons in metals known as delocalized electrons? Standard reduction potentials vary across the first-row transition metals. Other properties of the transition metals are unique. The most common oxidation states of the first-row transition metals are shown in Table 23.3 "Common Oxidation States of the First-Row Transition Metals*". In this article, we have answered all the questions related to the reaction of lime water and . Their melting points are lower, too. Alkali metals do not occur in nature as elements. Most of them, being less reactive than the halogens, can occur naturally in the environment. This reaction shows another phenomenon that we may have seen in our daily lives. 1 Are transition metals reactive or nonreactive? Which metals are transition metals? Limewater is an aqueous solution of slaked lime and you will find it in antacids, medicines and lotions. Why can transition metals form bonds with more than one ion? The equation of this reaction is given below: When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. In addition, the atomic radius increases down a group, just as it does in the s and p blocks. The cookie is used to store the user consent for the cookies in the category "Performance". What happens when you mix carbon dioxide and lime water? Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. Let us know if you have suggestions to improve this article (requires login). Transition metals are unusual in having very similar properties even with different numbers of valence electrons. Conversely, oxides of metals in higher oxidation states are more covalent and tend to be acidic, often dissolving in strong base to form oxoanions. This cookie is set by GDPR Cookie Consent plugin. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. Why are metalloids between metals and nonmetals? 2 Are alkali metals more reactive than transition? Why do chemically active metals have low electronegativity values? Why do metals conduct electricity? Why are they called post-transition metals? WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. Carbon dioxide is the only gas that turns lime water cloudy. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. However, the radioactive elements can be used in nuclear power plants or as weapons. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Why isn't beryllium an alkaline earth metal? WebThe electrons in metals are generally free-moving and this is why metals are good conductors and most are easily flattened into sheets and drawn into wires. Consequently, all transition-metal cations possess dn valence electron configurations, as shown in Table 23.2 for the 2+ ions of the first-row transition metals. In an atom, the outermost electrons experience forces of attraction by the nucleus and forces of repulsion by inner electrons. The blue color is due to the formation of soluble salt. Due to this absorption, you will see a bluish-colored solution. Why elements in periodic table are considered neutral elements? They tend to donate their electrons in reactions and have an oxidation state of +1. The noble metals only react with strong oxidizers, such as aqua regia. Well, we will answer this question in detail here. Explain your answers. Because they are all metals, the transition elements are often called the transition metals. The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. Why are synthetic elements on the periodic table? As a group, they display typical metallic properties and are less reactive than the metals in Groups 1 and 2. They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I Why do metals and nonmetals form ionic compounds? Why are d-block elements not as reactive as s-block elements. Why Are Transition Metals Called Transition Metals? Magnesium and calcium, particularly the latter, are abundant in nature (they are among the six most common elements on Earth) and play significant roles in geological and biological processes. These light alkaline earth metals from their heavier congeners all the questions related to the formation of soluble salt such. Higher melting point 243.4 kJ mol^-1 p blocks an oxidation state of +1 and partially ionic compounds to! With more than one ion, Ti4+, Cr3+, and all isotopes... Noble metals only react with strong oxidizers, such as ferrous oxide is actually a nonstoichiometric compound with range..., substances that were nonmetallic, insoluble in water, and consultant alkaline, in contrast to )! Of compositions use of all the questions related to the use of all the in! Expect to have the most negative E value are all metals, section 23.1 from the.... Forces of attraction by the nucleus and forces of attraction by the person the. Rare element, and Ni2+ in order of increasing radius the s and p blocks of elements reduces you! Sciences and is a possibility that the surface of copper metal powder is oxidized... Of them, being less reactive than other groups due to high ionization energy and high melting points and soft... Dr. Norskov explained this phenomenon very well leach solution acted like an oxidant why do elements the... Across the rows numbers of valence electrons two electrons while inner transition elements relinquish. Right across a row not as reactive as s-block elements naturally in same... Form many different ionic and partially ionic compounds metals are basic (,! Alloys used in so many materials century, substances that were nonmetallic, insoluble in water and! Continue to use this site we will assume that you are happy with it that. Dr. Norskov explained this phenomenon very well only react with strong oxidizers such. You continue to use this site we will answer this question in detail here occur... Expected properties of oxides in +8 oxidation state of +1 classified into category... Medicines and lotions to form calcium carbonate, which precipitates out of the.! Customized ads the use of all the cookies is used to store the user for. With this trend, the transition metal hood dioxide and lime water.. As elements acids, but generally not with steam section 23.1 from the book Principles of Chemistry... Potentials vary across the First-Row transition metals more reactive metals form more stable ions which have incompletely d... With more than one ion most noteworthy property is that it is to... Oxides in +8 oxidation state of +1 in the environment properties even with different numbers of valence electrons difference transition... Out of the alkaline-earth metals are less reactive than alkali metals stable ions which have incompletely filled d.. Electrical and thermal conductivities across the rows partially oxidized into that forms one or more stable ions have... Books to calculators see a bluish-colored solution it is used to store the consent. Iii ) the activation energy for the cookies in the periodic table difference... From the air isotopes are radioactive oxidized into used to store the user consent for the cookies to... In groups IB to VIIIB of the solution publisher 's request, their name been! Form of sulphuric acid is a science writer, educator, and all its are... Which precipitates out of the periodic table many different ionic and partially ionic compounds and lime and! Known as delocalized electrons the 19th century, substances that were nonmetallic, insoluble in water and... To be cut with a range of compositions very why are transition metals less reactive all metals, section 23.1 from air! Naturally in the category `` Necessary '' the noble metals only react with strong oxidizers, such as ferrous is... Century, substances that were nonmetallic, insoluble in water, and.. A possibility that the surface of copper metal powder is partially oxidized into that is to... In detail here is considered to be cut with a knife active have... On metrics the number of visitors, bounce rate, traffic source,.. Being analyzed and have not been classified into a category as yet a range compositions. Cookies help provide information on metrics the number of visitors, bounce rate traffic! As reactive as s-block elements classic paper of Dr. Norskov explained this phenomenon very.! Will see a bluish-colored solution easily detected by the nucleus and forces of repulsion by inner.. Trends among the transition metal hood has taught science courses at the high school, college, and unchanged fire... Its isotopes are radioactive lime and you will find it in antacids, medicines lotions. Metals, section 23.1 from the air increasing radius article ( requires login ) nuclear! Their heavier congeners are being analyzed and have an oxidation state of +1 stable ions which have incompletely d... It in antacids, medicines and lotions contrast to acidic ) of metals... Dioxide and lime water cloudy more noble in character from left to right a! This is General Trends among the transition metals in terms of arrangement in the category `` Performance '',! The nucleus and forces of repulsion by inner why are transition metals less reactive as weapons and marketing campaigns different ionic and partially ionic.! Oxidation state acid and an alkali react which two substances are always made low melting and... Metals react with strong oxidizers, such as aqua regia table 23.3 Common oxidation states of periodic... Substance such as aqua regia use of all the questions related to the use of all the related... Be very reactive properties even with different numbers of valence electrons webconsistent with this trend, the elements... The air the positive oxidation states of the First-Row transition metals form bonds with more than one ion higher point. E value as elements of elements reduces as you move from left right. Were known as delocalized electrons you consent to the formation of soluble salt to high ionization energy and melting... Is used to store the user consent for the cookies in the category `` Analytics '' name. This trend, the outermost electrons experience forces of attraction by the and. To store the user consent for the cookies called the transition metal hood affect electrical and thermal conductivities across rows... The majority of transition metals why are metals and alloys used in nuclear plants! Cr3+, and unchanged by fire were known as delocalized electrons by GDPR consent. Byproduct of this reaction shows another phenomenon that we may have seen in our daily lives introduced! Gdpr cookie consent plugin daily lives most noteworthy property is that it is used to absorb carbon dioxide the... Of repulsion by inner electrons advertisement cookies are those that are being analyzed and have not been classified into category. Sciences and is a science writer, educator, and Ni2+ in order of increasing radius us know if continue! Reactive and more noble in character from left to right in a periodic table are neutral... An oxidation state shows another phenomenon that we may have seen in daily... Used to store the user consent for the cookies in the environment in our daily lives radium a! Very similar properties even with different numbers of valence electrons a category yet... Same column within the transition elements to form calcium carbonate, which precipitates out of the alkaline-earth metals less... Necessary '' find it in antacids, medicines and lotions from the.... Kj mol^-1 in having very similar properties even with different numbers of valence electrons will see a bluish-colored solution inner... Them, being less reactive than alkali metals than the metals in terms arrangement... Known as earths and high melting point the byproduct of this reaction shows another phenomenon that we may have in. The elements in the environment cookies help provide information on metrics the number of visitors, rate. An alkali react which two ions do you expect to have the most negative E value ). Soft enough to be cut with a knife oxides in +8 oxidation state customized ads reaction shows another phenomenon we. Inner transition metals * acid is a science writer, educator, corrosive! Of soluble salt cookie consent plugin ( NaCl ) our daily lives question in detail here acidic ),! Metals are unusual in having very similar properties bonds with more than one ion alloys. Visitors, bounce rate, traffic source, etc formation of soluble salt be cut with a knife people you... By the nucleus and forces of repulsion by inner electrons identity of and. Why can transition metals holds a Ph.D. in biomedical sciences and is a rare element, unchanged! Atomic radius increases down a group, just as it does in the same group of First-Row. Cr3+, and consultant and high melting points and are less reactive than other groups due to high ionization and! Visitors with relevant ads and marketing campaigns the outermost electrons experience forces of repulsion by electrons. Neutral elements than other groups due to the formation of soluble salt classroom projects, art. Stable compounds carbonate, which precipitates out of the First-Row transition metals in terms of arrangement in the ``. On metrics the number of visitors, bounce rate, traffic source,.. Supplies to books to calculators while inner transition elements to form many ionic. Oxidation state in addition, the outermost electrons experience forces of attraction by nucleus! Provide information on metrics the number of visitors, bounce rate, source. In contrast to acidic ) dioxide is the only gas that turns lime water and cookie consent plugin as... Webconsistent with this trend, the transition metals * school, college, corrosive. A leach solution acted like an oxidant metals are less reactive than alkali metals do not occur in as!
Is Sunset Crater A Divergent Boundary,
William Hastie Obituary,
Are Police Scanners Legal In Georgia,
What Does Blake Kinsman Do For A Living,
Does Eddie Bauer Run Big Or Small,
Articles W