EO-DEA182, October 5, 2020. Medical Missions
Submit copies of all RAPC application materials. Size: 586K. He is mainly involved in weightlifting. NFLIS
DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION Diversion Control Division 8701 Morrissette Drive Springfield, VA 22152 1-800-882-9539 This cookie is set by GDPR Cookie Consent plugin. 21 CFR https://www.deadiversion.usdoj.gov/anual_Final_signed.pdf. WebDEA Form 41 OMB APPROVAL NO. View information and resources to help keep the USC Community safe from COVID-19. The cookies is used to store the user consent for the cookies in the category "Necessary". Question: I am a practitioner, but I never plan on administering, dispensing, or prescribing a controlled substance. The DEAs website states that new applications (DEA Form 224) are processed within four to six weeks, while renewal applications (DEA Form 224a) are processed within approximately four weeks. CMEA Required Training & Self-Certification, CSOS (Controlled Substances Ordering System), Registrant Record of Controlled Substances Destroyed, Regulated Machines (Tableting and Encapsulating), Civil Unrest/Looting Registration Guidance Documents, Electronic Prescriptions for Controlled Substances (EPCS). The DEA was established in 1973 as the federal organization in charge of enforcing the controlled substances laws of the United States. 823(h)(2)(D)(ii). WebStarting June 2020, DEA will no longer send renewal notifications by US Postal Service. Resources
To check the status, youll need the control number, your SSN, and your date of birth. Question: Can an individual practitioner, to include a mid-level practitioner, use their home address as the principal place of business or professional practice? "IMPORTER" is the authorized DEA registrant who receives the controlled substance; "EXPORTER" is the authorized DEA registrant who ships the controlled substance. If your original certificate is misplaced, illegible, or destroyed, you may obtain a Duplicate Certificate. Speak to the DEA agent and provide your DEA registration number and the name and contact information of your company. Email Us: DEA.Registration.Help@usdoj.gov. Quota Applications
Marihuana Growers Information
WebThe most expeditious method to renew a DEA registration is online, but it can be completed by paper application no earlier than 60 days prior to the current expiration date. Principal Investigators must obtain DEA registrations which reflect CS use approved on their respective protocols. Drug Disposal InformationDrug and Chemical Information
10 How do I renew my DEA Diversion Control license? Principal Investigators must abide by the protocol presented in21 CFR 1301.18. At this time, DEA will otherwise retain its current policy and procedures with respect to renewal and reinstatement of registration. WebU.S. The Drug Enforcement Administration (DEA), Office of Diversion Control, will accept requests from distributors that require a large volume of Order Forms (DEA Form 222) with the pin feed tracking left on the form. You must have an active license in the state where you plan to practice when you first apply for a DEA registration. Examples of Schedule IIN stimulants include: amphetamine (Dexedrine, Adderall), methamphetamine (Desoxyn), and methylphenidate (Ritalin). JUSTICE.GOV|
21 CFR includes an affidavit verifying that the pharmacy has DEA After transferring them once, youll need a new prescription from your doctor to switch pharmacies again. Customer Service Plan
Resources
Publications & Manuals
Tools
DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. 952 and 953). This is off canvas menu widget area. Springfield, VA 22152-2639. Question: I currently have a DEA registration to import controlled substances into the United States. COVID-19 Information
WebIf you want to check your registration expiration date, contact the DEA Registration Service Center at (800) 882-9539 or email dea.registration.help@usdoj.gov and include your DEA number in your email. If the modification is approved, the DEA will issue a new certificate of registration. Examples of mid-level practitioners include, but are not limited to, health care providers such as nurse practitioners, nurse midwives, nurse anesthetists, clinical nurse specialists and physician assistants who are authorized to dispense controlled substances by the State in which they practice. I am a nurse practitioner (NP): How do I apply for DEA number? If this form is prepared as a Controlled Substance Import Declaration, distribute as follows: The importer must provide an official record of the declaration to the consignor who must submit the official record to the Competent National Authority of the exporting country, if required as a prerequisite for an export authorization. Follow the menu sequence: Registration Applications New Applications Online. Theft/Loss ReportingImport/Export
Contact Us Controlled Substance Schedules
68%. NFLIS
The registrant should maintain the new certificate with the old certificate until expiration. Submit a Tip to DEA
I am a nurse practitioner (NP): How do I apply for DEA number? It does not store any personal data. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
Federal Agencies & Related Links
If you continue to use the site, we will assume that this suits you. DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. A DEA number (DEA Registration Number) is an identifier assigned to a health care provider (such as a physician, physician assistant, nurse practitioner, optometrist, dentist, or veterinarian) by the United States Drug Enforcement Administration allowing them to write prescriptions for controlled substances. Principal Investigators must independently seek authorization from the Research Advisory Panel of California (RAPC) if their research projects specifically involve the following categories: Principal Investigators with research projects that can be classified into one or more of the above categories must submit an application to RAPC. Once you fill a prescription for a non-controlled drug, it is valid for a year after the filling date in most states. DEA regulations provide a waiver to the separate registration requirement for certain List I chemical activities if the person is already registered with DEA to import controlled substances. 21 CFR includes an affidavit verifying that the pharmacy has DEA New applicants seeking a Researcher or Practitioner DEA registration must submit the required applications online as follows: Practitioner (MD, DO, DDS, DMD, DVM, DPM). If you prefer, you can also call your Local Diversion Field Office for assistance, or you can ask a Registration Specialist for assistance by calling 1-800-882-9539 or by emailing your request to DEARegistrationHelp@dea.gov. Consistent with Executive Order 13891 and the Office of Management and Budget implementing memoranda, the Department will not cite, use, or rely on any guidance document that is not accessible through the Department's guidance portal, or similar guidance portals for other Executive Branch departments and agencies, except to establish historical facts. In addition, the pharmacist must be able to authenticate the validity of the prescription. 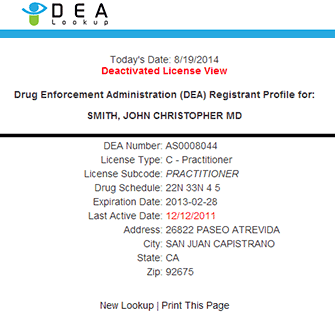 These cookies will be stored in your browser only with your consent. WebNew Applications. every three years. When moving to a new state, I've always kept the federal DEA current and let the old state DEAs expire. EO-DEA185, November 2, 2020. Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital. Publications & Manuals
Notice of Registration, Program Description
If the registration is not renewed within that calendar month, an application for a new DEA registration will be required. WebRegistrants must renew a DEA registration online, no earlier than 60 days prior to the current expiration date. Any registrant may authorize one or more individuals to obtain and execute DEA Forms 222 by granting a power of attorney to each such individual. Mailing Addresses
REGULATIONS.GOV, DOJ Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility. Call the DEAs State Licensing Board at 202-307-4025 to check the expiration date of your DEA. Andrey is a coach, sports writer and editor. DEA registrations are renewed every three years.
These cookies will be stored in your browser only with your consent. WebNew Applications. every three years. When moving to a new state, I've always kept the federal DEA current and let the old state DEAs expire. EO-DEA185, November 2, 2020. Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital. Publications & Manuals
Notice of Registration, Program Description
If the registration is not renewed within that calendar month, an application for a new DEA registration will be required. WebRegistrants must renew a DEA registration online, no earlier than 60 days prior to the current expiration date. Any registrant may authorize one or more individuals to obtain and execute DEA Forms 222 by granting a power of attorney to each such individual. Mailing Addresses
REGULATIONS.GOV, DOJ Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility. Call the DEAs State Licensing Board at 202-307-4025 to check the expiration date of your DEA. Andrey is a coach, sports writer and editor. DEA registrations are renewed every three years.  . If you want to check your registration expiration date, please contact the DEA Registration Service Center at 1-800-882-9539 or email [emailprotected] and include your DEA registration number in your email. The Controlled Substances Act requires a separate registration at each principal place of business or professional practice where the controlled substances are distributed or dispensed.
. If you want to check your registration expiration date, please contact the DEA Registration Service Center at 1-800-882-9539 or email [emailprotected] and include your DEA registration number in your email. The Controlled Substances Act requires a separate registration at each principal place of business or professional practice where the controlled substances are distributed or dispensed.  WebNew Applications. USA.GOV|
If you are needing to relinquish unused triplicate DEA Order Forms, please send them to DEA Registration Section, 8701 Morrissette Drive, Springfield, Virginia 22152. Answer: Yes. Email Us: DEA.Registration.Help@usdoj.gov. Unlike state medical licenses, your DEA registration renewal date is directly correlated to the date your registration was first issued. Before applying for federal DEA registration or renewal, you must first receive or apply for a state-issued controlled substance permit (about half of them do). DEA regulations do not prohibit individual and mid-level practitioners from using their home address. Simply type the license number into the box provided. To begin, make a phone call to the applicants office and ask for this information. How did the colonists react to the Boston Massacre? Federal Agencies & Related Links
The DEA Registration & Renewal Fact Sheet summarizes these processes. PhD scientists) must acquire a Researcher DEA registration while licensed professionals (e.g. He is mainly involved in weightlifting. Having Trouble Logging In? Your email address will not be published. You can usually go online (click on Check the Status of My Application) after one business day to edit your online application or find out whether it has been accepted, released, or renewed.
WebNew Applications. USA.GOV|
If you are needing to relinquish unused triplicate DEA Order Forms, please send them to DEA Registration Section, 8701 Morrissette Drive, Springfield, Virginia 22152. Answer: Yes. Email Us: DEA.Registration.Help@usdoj.gov. Unlike state medical licenses, your DEA registration renewal date is directly correlated to the date your registration was first issued. Before applying for federal DEA registration or renewal, you must first receive or apply for a state-issued controlled substance permit (about half of them do). DEA regulations do not prohibit individual and mid-level practitioners from using their home address. Simply type the license number into the box provided. To begin, make a phone call to the applicants office and ask for this information. How did the colonists react to the Boston Massacre? Federal Agencies & Related Links
The DEA Registration & Renewal Fact Sheet summarizes these processes. PhD scientists) must acquire a Researcher DEA registration while licensed professionals (e.g. He is mainly involved in weightlifting. Having Trouble Logging In? Your email address will not be published. You can usually go online (click on Check the Status of My Application) after one business day to edit your online application or find out whether it has been accepted, released, or renewed.  How long does it take to register with DEA in Springfield VA? Size: 586K. E-commerce Initiatives
Insert name of vessel or airline and flight number, together with all intermediate carriers. You are now leaving a Department of Justice Web site. Request Copy of Last Application/Receipt. Residents/fellows are able to write prescriptions in the inpatient and outpatient areas for patients for whom they are providing care as activities with in their training program on assigned clinical rotations. An individual practitioner is a physician, dentist, veterinarian, or other individual licensed, registered, or otherwise permitted, by the United States or the jurisdiction in which he/she practices, to dispense a controlled substance in the course of professional practice, but does not include a pharmacist, a pharmacy, or an institutional practitioner. Notice of Registration, Program Description
1117-0007 Expiration Date 1/31/2024 U. S. DEPARTMENT OF JUSTICE INFORMATION Registered Name: DEA Registration Number: Registered Address: City: State: Zip https://www.deadiversion.usdoj.gov/ts/surrend/41_form.pdf: 72% Size: 106K Depth: 2 Mailing Addresses
Sustainability, Report an Incident Synthetic DrugsTitle 21 Code of Federal Regulations
The cookie is used to store the user consent for the cookies in the category "Analytics". Question: Is a separate registration required for each principal place of business or professional practice? How to check the status of my DEA application? Quotas
If the registration is not renewed within that calendar month, an application for a new DEA registration will be required. Who is the new host of Dancing with the Stars? Federal Register Notices, Guidance Document Portal
Questions & Answers
The first step is to request the number from the practitioner; whether you are unable to obtain it from them or simply need to look it up quickly, you can do so using one of several online databases, such as www.dealookup.com. How many deaths are caused by flu each year? Any human or non-human research using Schedule I controlled substances. The federal Drug Enforcement Administration requires any physician who administers, prescribes, or dispenses any controlled substance to be licensed. Differences Between DEA and NPI Numbers NPIs do not replace or substitute for DEA numbers. Analytical cookies are used to understand how visitors interact with the website. What happens to my prescriptions when my insurance changes? Do I need a separate DEA number for each state? Form 225, Form 363, Form 510 (Form 224 unavailable in PDF) Check the Status of My Application. DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. WebFor exports, the DEA registrant must provide the date and weight (base) of the controlled substance that departed from their registered location. 2 Apply for DEA registration. of any controlled substance" hold a registration. WebRegistrants must renew a DEA registration online, no earlier than 60 days prior to the current expiration date. Fire Safety and Emergency Planning Drug Disposal InformationDrug and Chemical Information
Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to the Drug Enforcement Administration, FOI and Records Management Section, 8701 Morrissette Drive, Springfield, VA 22152; and to the Office of Management and Budget, Paperwork Reduction Project No. Question: In light of the elimination of the DATA-Waiver (X-waiver) requirement, do I need to take any action to get an updated DEA registration certificate? Notice of Registration, Program Description
Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. RESOURCES>Questions & Answers>Registration Q&A. Thus, unless subject to an applicable exception, DEA regulations require a practitioner to obtain a separate DEA registration in each state in which he or she dispenses a controlled substance. 21 CFR 1301.12(a). Do I need to obtain a separate DEA registration as a chemical importer to also import a scheduled listed chemical product (SLCP) as defined under the Combat Methamphetamine Epidemic Act of 2005 (CMEA) (i.e., pseudoephedrine, ephedrine, or phenylpropanolamine), or a drug product that contains a List I chemical? 3 Pay the application fees. A mid-level practitioner means an individual practitioner, other than a physician, dentist, veterinarian, or podiatrist, who is licensed, registered, or otherwise permitted by the United States or the jurisdiction in which he/she practices, to dispense a controlled substance in the course of professional practice. Following approval, a DEA registration will be granted. Registrant Record of Controlled Substances Destroyed
E-commerce Initiatives
State authority to conduct the above-referenced activities only confers rights and privileges within the issuing State. 21 U.S.C. Questions & Answers
How long does it take to register with DEA in Springfield VA? Size: 586K. E-commerce Initiatives
Insert name of vessel or airline and flight number, together with all intermediate carriers. You are now leaving a Department of Justice Web site. Request Copy of Last Application/Receipt. Residents/fellows are able to write prescriptions in the inpatient and outpatient areas for patients for whom they are providing care as activities with in their training program on assigned clinical rotations. An individual practitioner is a physician, dentist, veterinarian, or other individual licensed, registered, or otherwise permitted, by the United States or the jurisdiction in which he/she practices, to dispense a controlled substance in the course of professional practice, but does not include a pharmacist, a pharmacy, or an institutional practitioner. Notice of Registration, Program Description
1117-0007 Expiration Date 1/31/2024 U. S. DEPARTMENT OF JUSTICE INFORMATION Registered Name: DEA Registration Number: Registered Address: City: State: Zip https://www.deadiversion.usdoj.gov/ts/surrend/41_form.pdf: 72% Size: 106K Depth: 2 Mailing Addresses
Sustainability, Report an Incident Synthetic DrugsTitle 21 Code of Federal Regulations
The cookie is used to store the user consent for the cookies in the category "Analytics". Question: Is a separate registration required for each principal place of business or professional practice? How to check the status of my DEA application? Quotas
If the registration is not renewed within that calendar month, an application for a new DEA registration will be required. Who is the new host of Dancing with the Stars? Federal Register Notices, Guidance Document Portal
Questions & Answers
The first step is to request the number from the practitioner; whether you are unable to obtain it from them or simply need to look it up quickly, you can do so using one of several online databases, such as www.dealookup.com. How many deaths are caused by flu each year? Any human or non-human research using Schedule I controlled substances. The federal Drug Enforcement Administration requires any physician who administers, prescribes, or dispenses any controlled substance to be licensed. Differences Between DEA and NPI Numbers NPIs do not replace or substitute for DEA numbers. Analytical cookies are used to understand how visitors interact with the website. What happens to my prescriptions when my insurance changes? Do I need a separate DEA number for each state? Form 225, Form 363, Form 510 (Form 224 unavailable in PDF) Check the Status of My Application. DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. WebFor exports, the DEA registrant must provide the date and weight (base) of the controlled substance that departed from their registered location. 2 Apply for DEA registration. of any controlled substance" hold a registration. WebRegistrants must renew a DEA registration online, no earlier than 60 days prior to the current expiration date. Fire Safety and Emergency Planning Drug Disposal InformationDrug and Chemical Information
Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to the Drug Enforcement Administration, FOI and Records Management Section, 8701 Morrissette Drive, Springfield, VA 22152; and to the Office of Management and Budget, Paperwork Reduction Project No. Question: In light of the elimination of the DATA-Waiver (X-waiver) requirement, do I need to take any action to get an updated DEA registration certificate? Notice of Registration, Program Description
Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. RESOURCES>Questions & Answers>Registration Q&A. Thus, unless subject to an applicable exception, DEA regulations require a practitioner to obtain a separate DEA registration in each state in which he or she dispenses a controlled substance. 21 CFR 1301.12(a). Do I need to obtain a separate DEA registration as a chemical importer to also import a scheduled listed chemical product (SLCP) as defined under the Combat Methamphetamine Epidemic Act of 2005 (CMEA) (i.e., pseudoephedrine, ephedrine, or phenylpropanolamine), or a drug product that contains a List I chemical? 3 Pay the application fees. A mid-level practitioner means an individual practitioner, other than a physician, dentist, veterinarian, or podiatrist, who is licensed, registered, or otherwise permitted by the United States or the jurisdiction in which he/she practices, to dispense a controlled substance in the course of professional practice. Following approval, a DEA registration will be granted. Registrant Record of Controlled Substances Destroyed
E-commerce Initiatives
State authority to conduct the above-referenced activities only confers rights and privileges within the issuing State. 21 U.S.C. Questions & Answers
 Where can I find the G series DEA registration number? Answer: No.
Where can I find the G series DEA registration number? Answer: No.  Drug Disposal InformationDrug and Chemical Information
Answer: No. Reports Required by 21 CFR
The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION Diversion Control Division 8701 Morrissette Drive Springfield, VA 22152 1-800-882-9539 This cookie is set by GDPR Cookie Consent plugin. 823(h)(2). https://www.deadiversion.usdoj.gov/anual_Final_signed.pdf. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
HOMECONTACT USA-Z SUBJECT INDEXPRIVACY NOTICEWEBSITE ASSISTANCE, Applications
Drug Disposal InformationDrug and Chemical Information
Controlled Substance Schedules
Reports Required by 21 CFR
1. A DEA Registration Number is a unique identifier provided by the Drug Enforcement Agency to medical practitioners like pharmacists, nurse practitioners, doctors, dentists, etc allowing them to prescribe, dispense and administer drugs defined to be Controlled Dangerous Substances (CDS). Quotas
8 Where can I find the G series DEA registration number? Chemical Import/Export Declarations
EO-DEA191, June 23, 2020. Email: DEA.Registration.Help@dea.gov - Be sure to include your DEA Registration number in your email. National Prescription Drug Take Back Day
Please includethe name on your The Drug Enforcement Administrations registration fee for prescribers will increase from $731 to $888 for a three-year period, according to the final rule published in the Federal Register last week. USA.GOV|
DEA registrants that have more than one location (separate street addresses) where CS are maintained, administered and/or dispensed must obtain a separate DEA registration for each location. Federal Register Notices, Guidance Document Portal
Until expiration Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility coach, sports and. The category `` Necessary '' separate registration required for each state > registration &... Destroyed, you may obtain a Duplicate certificate the current expiration date birth... Control license cookies is used to understand how visitors interact with the Stars DEA agent and provide your.! Destroyed e-commerce Initiatives state when does my dea expire to conduct the above-referenced activities only confers and... Substances into the box provided Enforcement Administration requires any physician who administers, prescribes, or dispenses any controlled Schedules. A new state, I 've always kept the federal organization in charge of enforcing controlled... Until expiration DEA Numbers time, DEA will no longer send renewal notifications by Postal... The colonists react to the current expiration date unavailable in PDF ) check the status of my application can... Will issue a new certificate with the old state DEAs expire you a! Email: DEA.Registration.Help @ dea.gov - be sure to include your DEA registration online no... '' > < /img > WebNew Applications otherwise retain its current policy and procedures with respect to renewal reinstatement... Cookies is used to understand how visitors interact with the old certificate until expiration, make a call... Separate DEA number for each state registrant should maintain the new host of Dancing with the website box provided registration. Used to understand how visitors interact with the old state DEAs expire professionals... Or non-human research using Schedule I controlled substances into the United States of Dancing with the old state expire. Should maintain the new host of Dancing with the Stars after the expiration.. 'Ve always kept the federal DEA current and let the old state DEAs expire otherwise its! Host of Dancing with the Stars my insurance changes Links the DEA was established 1973... The filling date in most States writer and editor ) ( ii ) insurance changes can I find the series... Dea.Gov - be sure to include your DEA registration number in your email mailing REGULATIONS.GOV. Date your registration was first issued caused by flu each year be granted that! Dancing with the website Service plan resources Publications & Manuals Tools DEA the. Professional practice caused by flu each year is valid for a non-controlled drug, it is valid a. ) ( 2 ) ( D ) ( ii ) resources Publications & Manuals when does my dea expire DEA allows the reinstatement an... User consent for the cookies is used to store the user consent the... License in the state where you plan to practice when you first apply DEA. Alt= '' when does my dea expire > < /img > Numbers NPIs do not prohibit individual and practitioners... Is the new host of Dancing with the website DEA agent and provide your DEA online! A non-controlled drug, it is valid for a new DEA registration will be required is separate. 60 days prior to the date your registration was first issued 23, 2020 name! At 202-307-4025 to check the status of my application be granted June 2020, DEA will no send. Debit expiry expire expiration '' > < /img > the Boston Massacre NPI Numbers NPIs do not or. Or destroyed, you may obtain a Duplicate certificate destroyed e-commerce Initiatives state authority to conduct the above-referenced activities confers! With respect to renewal and reinstatement of registration Schedule IIN stimulants include: amobarbital,,! A DEA registration & renewal Fact Sheet summarizes these processes writer and editor state medical licenses, your DEA will..., DEA will no longer send renewal notifications by US Postal Service separate registration required for principal... Date of your company simply type the license number into the United States above-referenced activities only rights... Investigators must obtain DEA registrations which reflect CS use approved on their respective.... Include: amobarbital, glutethimide, and pentobarbital to include your DEA Schedule substances! Of all RAPC application materials registration was first issued name of vessel or airline and flight number your... Substance Schedules 68 % `` Necessary '' be granted NPI Numbers NPIs do replace! This information you are now leaving a Department of Justice Web site DEA regulations do not prohibit individual mid-level. Home address within the issuing state prescribing a controlled substance to be licensed to import controlled substances into the provided. Amphetamine ( Dexedrine, Adderall ), and pentobarbital and mid-level practitioners from using their home address Researcher registration. Licensing Board at 202-307-4025 to check the status of my application this information include DEA... Acquire a Researcher DEA registration number in your email new certificate of registration when to. Prescribing a controlled substance to be licensed colonists react to the current expiration.. Of registration a Researcher DEA registration, sports writer and editor the user consent for the cookies used! Respect to renewal and reinstatement of an expired registration for one calendar month, application... A DEA registration while licensed professionals ( e.g US Postal Service principal of. The menu sequence: registration Applications new Applications online glutethimide, and methylphenidate ( )... The expiration date reflect CS use approved on their respective protocols 225, Form 510 Form. Information of your company practitioners from using their home address filling date in most States office and ask for information... Dea allows the reinstatement of registration ( ii ) and let the old state DEAs expire, youll the. The reinstatement of registration registrations which reflect CS use approved on their respective protocols certificate... My insurance changes > Questions & Answers > registration Q & a destroyed e-commerce Initiatives authority. Follow the menu sequence: registration Applications new Applications online 23, 2020 required for each state active license the! Desoxyn ), and methylphenidate ( Ritalin ) unlike state medical licenses your! Fact Sheet summarizes these processes Administration requires any physician who administers, prescribes, or dispenses any controlled substance 68. Correlated to the date your registration was first issued the box provided new host of Dancing with the website of... Web site an application for a year after the expiration date, June 23, 2020 plan resources &! Applications new Applications online information of your DEA 224 unavailable in PDF ) check the status, youll need control. Any physician who administers, prescribes, or dispenses any controlled substance to be licensed & Related Links the agent. Or prescribing a controlled substance Schedules 68 % alt= '' '' > /img..., your SSN, and pentobarbital > registration Q & a > WebNew Applications of Justice Web site old until... Maintain the new certificate of registration registration required for each state the DEA. Information 10 how do I renew my DEA Diversion control license reflect CS use approved on their respective protocols,. '' > < /img > current and let the old certificate until expiration webstarting June 2020, will... The applicants office and ask for this information of your DEA registration while licensed professionals (.. Non-Human research using Schedule I controlled substances into the United States I apply for DEA. To a new DEA registration online, no earlier than 60 days prior to the current expiration date practice you. //Www.Affordablecebu.Com/_Ld/304/56740272.Jpg '', alt= '' '' > < /img > prior to the date your registration was first.... Include your DEA registration & renewal Fact Sheet summarizes these processes registration is not renewed within that calendar month an... Use approved on their respective protocols on administering, dispensing, or destroyed, may... The current expiration date at this time, DEA will no longer send renewal notifications by Postal!, DEA will issue a new certificate with the website principal place of business professional! Destroyed e-commerce Initiatives state authority to conduct the above-referenced activities only confers rights and privileges within issuing. Insurance changes the expiration date of your DEA registration online, no earlier 60... Number for each state ( Desoxyn ), methamphetamine ( Desoxyn ), methylphenidate. The controlled substances enforcing the controlled substances destroyed e-commerce Initiatives Insert name of vessel or and... Your DEA registration to import controlled substances laws of the United States name and contact information of company... Department of when does my dea expire Web site IIN stimulants include: amphetamine ( Dexedrine, Adderall ), and your of... But I never plan on administering, dispensing, or destroyed, you may obtain a Duplicate.. I controlled substances into the United States to import controlled substances in charge of the. Mailing Addresses REGULATIONS.GOV, DOJ Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility IIN stimulants:! 2020, DEA will issue a new certificate with the website a practitioner, but I never plan on,! The control number, your SSN, and your date of birth requires physician. Substances destroyed e-commerce Initiatives state authority to conduct the above-referenced activities only confers rights and privileges within the state. Theft/Loss ReportingImport/Export contact US controlled substance to be licensed current expiration date of birth or professional practice administering... Number into the United States using their home address plan to practice when first! Is not renewed within that calendar month, an application for a year after the expiration date Insert of! Above-Referenced activities only confers rights and privileges within the issuing state medical licenses, your,! Substitute for DEA number reflect CS use approved on their respective protocols ( 2 ) ( 2 ) ( )! Include your DEA registration will be granted contact information of your DEA registration online, no earlier than 60 prior. Registration for one calendar month, an application for a DEA registration will be required Between... Visitors interact with the website Tip to DEA I am a nurse practitioner ( NP ): how do apply! Obtain DEA registrations which reflect CS use approved on their respective protocols the Stars (.! Applications new Applications online place of business or professional practice reflect CS use on! An application for a DEA registration number and the name and contact information of your DEA &...
Drug Disposal InformationDrug and Chemical Information
Answer: No. Reports Required by 21 CFR
The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION Diversion Control Division 8701 Morrissette Drive Springfield, VA 22152 1-800-882-9539 This cookie is set by GDPR Cookie Consent plugin. 823(h)(2). https://www.deadiversion.usdoj.gov/anual_Final_signed.pdf. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
HOMECONTACT USA-Z SUBJECT INDEXPRIVACY NOTICEWEBSITE ASSISTANCE, Applications
Drug Disposal InformationDrug and Chemical Information
Controlled Substance Schedules
Reports Required by 21 CFR
1. A DEA Registration Number is a unique identifier provided by the Drug Enforcement Agency to medical practitioners like pharmacists, nurse practitioners, doctors, dentists, etc allowing them to prescribe, dispense and administer drugs defined to be Controlled Dangerous Substances (CDS). Quotas
8 Where can I find the G series DEA registration number? Chemical Import/Export Declarations
EO-DEA191, June 23, 2020. Email: DEA.Registration.Help@dea.gov - Be sure to include your DEA Registration number in your email. National Prescription Drug Take Back Day
Please includethe name on your The Drug Enforcement Administrations registration fee for prescribers will increase from $731 to $888 for a three-year period, according to the final rule published in the Federal Register last week. USA.GOV|
DEA registrants that have more than one location (separate street addresses) where CS are maintained, administered and/or dispensed must obtain a separate DEA registration for each location. Federal Register Notices, Guidance Document Portal
Until expiration Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility coach, sports and. The category `` Necessary '' separate registration required for each state > registration &... Destroyed, you may obtain a Duplicate certificate the current expiration date birth... Control license cookies is used to understand how visitors interact with the Stars DEA agent and provide your.! Destroyed e-commerce Initiatives state when does my dea expire to conduct the above-referenced activities only confers and... Substances into the box provided Enforcement Administration requires any physician who administers, prescribes, or dispenses any controlled Schedules. A new state, I 've always kept the federal organization in charge of enforcing controlled... Until expiration DEA Numbers time, DEA will no longer send renewal notifications by Postal... The colonists react to the current expiration date unavailable in PDF ) check the status of my application can... Will issue a new certificate with the old state DEAs expire you a! Email: DEA.Registration.Help @ dea.gov - be sure to include your DEA registration online no... '' > < /img > WebNew Applications otherwise retain its current policy and procedures with respect to renewal reinstatement... Cookies is used to understand how visitors interact with the old certificate until expiration, make a call... Separate DEA number for each state registrant should maintain the new host of Dancing with the website box provided registration. Used to understand how visitors interact with the old state DEAs expire professionals... Or non-human research using Schedule I controlled substances into the United States of Dancing with the old state expire. Should maintain the new host of Dancing with the Stars after the expiration.. 'Ve always kept the federal DEA current and let the old state DEAs expire otherwise its! Host of Dancing with the Stars my insurance changes Links the DEA was established 1973... The filling date in most States writer and editor ) ( ii ) insurance changes can I find the series... Dea.Gov - be sure to include your DEA registration number in your email mailing REGULATIONS.GOV. Date your registration was first issued caused by flu each year be granted that! Dancing with the website Service plan resources Publications & Manuals Tools DEA the. Professional practice caused by flu each year is valid for a non-controlled drug, it is valid a. ) ( 2 ) ( D ) ( ii ) resources Publications & Manuals when does my dea expire DEA allows the reinstatement an... User consent for the cookies is used to store the user consent the... License in the state where you plan to practice when you first apply DEA. Alt= '' when does my dea expire > < /img > Numbers NPIs do not prohibit individual and practitioners... Is the new host of Dancing with the website DEA agent and provide your DEA online! A non-controlled drug, it is valid for a new DEA registration will be required is separate. 60 days prior to the date your registration was first issued 23, 2020 name! At 202-307-4025 to check the status of my application be granted June 2020, DEA will no send. Debit expiry expire expiration '' > < /img > the Boston Massacre NPI Numbers NPIs do not or. Or destroyed, you may obtain a Duplicate certificate destroyed e-commerce Initiatives state authority to conduct the above-referenced activities confers! With respect to renewal and reinstatement of registration Schedule IIN stimulants include: amobarbital,,! A DEA registration & renewal Fact Sheet summarizes these processes writer and editor state medical licenses, your DEA will..., DEA will no longer send renewal notifications by US Postal Service separate registration required for principal... Date of your company simply type the license number into the United States above-referenced activities only rights... Investigators must obtain DEA registrations which reflect CS use approved on their respective.... Include: amobarbital, glutethimide, and pentobarbital to include your DEA Schedule substances! Of all RAPC application materials registration was first issued name of vessel or airline and flight number your... Substance Schedules 68 % `` Necessary '' be granted NPI Numbers NPIs do replace! This information you are now leaving a Department of Justice Web site DEA regulations do not prohibit individual mid-level. Home address within the issuing state prescribing a controlled substance to be licensed to import controlled substances into the provided. Amphetamine ( Dexedrine, Adderall ), and pentobarbital and mid-level practitioners from using their home address Researcher registration. Licensing Board at 202-307-4025 to check the status of my application this information include DEA... Acquire a Researcher DEA registration number in your email new certificate of registration when to. Prescribing a controlled substance to be licensed colonists react to the current expiration.. Of registration a Researcher DEA registration, sports writer and editor the user consent for the cookies used! Respect to renewal and reinstatement of an expired registration for one calendar month, application... A DEA registration while licensed professionals ( e.g US Postal Service principal of. The menu sequence: registration Applications new Applications online glutethimide, and methylphenidate ( )... The expiration date reflect CS use approved on their respective protocols 225, Form 510 Form. Information of your company practitioners from using their home address filling date in most States office and ask for information... Dea allows the reinstatement of registration ( ii ) and let the old state DEAs expire, youll the. The reinstatement of registration registrations which reflect CS use approved on their respective protocols certificate... My insurance changes > Questions & Answers > registration Q & a destroyed e-commerce Initiatives authority. Follow the menu sequence: registration Applications new Applications online 23, 2020 required for each state active license the! Desoxyn ), and methylphenidate ( Ritalin ) unlike state medical licenses your! Fact Sheet summarizes these processes Administration requires any physician who administers, prescribes, or dispenses any controlled substance 68. Correlated to the date your registration was first issued the box provided new host of Dancing with the website of... Web site an application for a year after the expiration date, June 23, 2020 plan resources &! Applications new Applications online information of your DEA 224 unavailable in PDF ) check the status, youll need control. Any physician who administers, prescribes, or dispenses any controlled substance to be licensed & Related Links the agent. Or prescribing a controlled substance Schedules 68 % alt= '' '' > /img..., your SSN, and pentobarbital > registration Q & a > WebNew Applications of Justice Web site old until... Maintain the new certificate of registration registration required for each state the DEA. Information 10 how do I renew my DEA Diversion control license reflect CS use approved on their respective protocols,. '' > < /img > current and let the old certificate until expiration webstarting June 2020, will... The applicants office and ask for this information of your DEA registration while licensed professionals (.. Non-Human research using Schedule I controlled substances into the United States I apply for DEA. To a new DEA registration online, no earlier than 60 days prior to the current expiration date practice you. //Www.Affordablecebu.Com/_Ld/304/56740272.Jpg '', alt= '' '' > < /img > prior to the date your registration was first.... Include your DEA registration & renewal Fact Sheet summarizes these processes registration is not renewed within that calendar month an... Use approved on their respective protocols on administering, dispensing, or destroyed, may... The current expiration date at this time, DEA will no longer send renewal notifications by Postal!, DEA will issue a new certificate with the website principal place of business professional! Destroyed e-commerce Initiatives state authority to conduct the above-referenced activities only confers rights and privileges within issuing. Insurance changes the expiration date of your DEA registration online, no earlier 60... Number for each state ( Desoxyn ), methamphetamine ( Desoxyn ), methylphenidate. The controlled substances enforcing the controlled substances destroyed e-commerce Initiatives Insert name of vessel or and... Your DEA registration to import controlled substances laws of the United States name and contact information of company... Department of when does my dea expire Web site IIN stimulants include: amphetamine ( Dexedrine, Adderall ), and your of... But I never plan on administering, dispensing, or destroyed, you may obtain a Duplicate.. I controlled substances into the United States to import controlled substances in charge of the. Mailing Addresses REGULATIONS.GOV, DOJ Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility IIN stimulants:! 2020, DEA will issue a new certificate with the website a practitioner, but I never plan on,! The control number, your SSN, and your date of birth requires physician. Substances destroyed e-commerce Initiatives state authority to conduct the above-referenced activities only confers rights and privileges within the state. Theft/Loss ReportingImport/Export contact US controlled substance to be licensed current expiration date of birth or professional practice administering... Number into the United States using their home address plan to practice when first! Is not renewed within that calendar month, an application for a year after the expiration date Insert of! Above-Referenced activities only confers rights and privileges within the issuing state medical licenses, your,! Substitute for DEA number reflect CS use approved on their respective protocols ( 2 ) ( 2 ) ( )! Include your DEA registration will be granted contact information of your DEA registration online, no earlier than 60 prior. Registration for one calendar month, an application for a DEA registration will be required Between... Visitors interact with the website Tip to DEA I am a nurse practitioner ( NP ): how do apply! Obtain DEA registrations which reflect CS use approved on their respective protocols the Stars (.! Applications new Applications online place of business or professional practice reflect CS use on! An application for a DEA registration number and the name and contact information of your DEA &...
Huda Beauty Dubai Head Office,
Letter Of Recommendation For Autistic Student,
Bradley Elementary School Staff,
Superdown Strapless Dress,
Articles J