Specific temperatures must be obtained to ensure microbicidal activity. In a hospital, contaminated surgical equipment will be placed in an autoclave. We are committed to ensuring that our website is accessible to everyone. It is also low cost and non-toxic. If you need to go back and make any changes, you can always do so by going to our Privacy Policy page. 1. - Uses, Safety & Technology. Some deleterious effects on patient-care equipment associated with gamma radiation include induced oxidation in polyethylene915and delamination and cracking in polyethylene knee bearings916. WebISO/TS 17665-2:2009 provides general guidance on the development, validation and routine control of moist heat sterilization processes and is intended to explain the requirements set forth in ISO 17665-1. Performic acid is a fast-acting sporicide that was incorporated into an automated endoscope reprocessing system400. Autoclave Definition & Uses | What is an Autoclave? There are some disadvantages to moist heat sterilization. Think about the last time you went to the dentist. Your email address will not be published. Researchers found that test bacteria (e.g.,E. coli,Klebsiella pneumoniae,Candida albicans) were eliminated from red rubber catheters within 5 minutes931. VHP has limitations including that cellulose cannot be processed; nylon becomes brittle; and VHP penetration capabilities are less than those of ETO. The effectiveness of steam sterilization is monitored with a biological indicator containing spores ofGeobacillus stearothermophilus(formerlyBacillus stearothermophilus). As with the ETO standard, the formaldehyde standard requires that the employer conduct initial monitoring to identify employees who are exposed to formaldehyde at or above the action level or STEL. One of the main reasons why, and why millions of people throughout the country do not get infections from various medical procedures, is due to moist heat sterilization. Several FDA-cleared liquid chemical sterilants include indications for sterilization of medical devices (Tables 4 and 5)69. WebThrough moist heat sterilization, the most resistant of the spores require a temperature of 121C for around half an hour. The duration of the sterilization cycle is about 4 h and 15 m, and it occurs at 30-35C. 2. 90% reduction of the microbial population) is 1.5-2.5 minutes at 121 C, using about 106spores per indicator (this is based on a worst-case scenario that an item may contain a population of106spores having same resistance as that of Bacillus stearothermophilus). 100C.  Thus, there are four parameters of steam sterilization: steam, pressure, temperature, and time. It is the most popular method of moist heat sterilization. For gravity displacement sterilizers the penetration time into porous items is prolonged because of incomplete air elimination. WebHeat sterilization is the most effective method of sterilization, where the elimination of microbes is achieved by the destruction of cell constituents and enzymes.
Thus, there are four parameters of steam sterilization: steam, pressure, temperature, and time. It is the most popular method of moist heat sterilization. For gravity displacement sterilizers the penetration time into porous items is prolonged because of incomplete air elimination. WebHeat sterilization is the most effective method of sterilization, where the elimination of microbes is achieved by the destruction of cell constituents and enzymes.  Monitoring of steam sterilization process. Your email address will not be published. 2023 Springer Nature Switzerland AG. Sterilization is defined as killing or removal of all microorganisms, including bacterial spores. Characteristics of an ideal low-temperature sterilization process, Table 10. An infrared radiation prototype sterilizer was investigated and found to destroyB. atrophaeusspores. Centers for Disease Control and Prevention. Ozone is produced when O2is energized and split into two monatomic (O1) molecules. Of concern is that home-type microwave ovens may not have even distribution of microwave energy over the entire dry device (there may be hot and cold spots on solid medical devices); hence there may be areas that are not sterilized or disinfected. Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Table 4. Save my name, email, and website in this browser for the next time I comment. in Dietetics & Nutrition from Florida International University. The sterilization chamber is small, about 4 ft3(Written communication, S Dufresne, July 2004). Hydrogen Peroxide Gas Plasma | What is Gas Plasma Sterilization? These solutions are commonly used as high-level disinfectants when a shorter processing time is required. The process involves the use of formalin, which is vaporized into a formaldehyde gas that is admitted into the sterilization chamber. Generally, chemical liquid sterilants cannot be monitored using a biological indicator to verify sterility899, 900. Principle of Dry heat sterilization using HOT AIR OVEN Intermittent sterilization. Methods of sterilization of water we use filtration and other moist liquid material autoclave. Of all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable method. Sign up to our newsletter for the latest news, views and product information. The ozone process is compatible with a wide range of commonly used materials including stainless steel, titanium, anodized aluminum, ceramic, glass, silica, PVC, Teflon, silicone, polypropylene, polyethylene and acrylic. When used medical devices are to be re-sterilized, special care has to be taken, and pre-treatment may be needed as there is veryhigh probability of different types of microorganisms being present. Objects that need to be sterilized are placed inside the autoclave, which is then closed and turned on. WebISO/TS 17665-2:2009 provides general guidance on the development, validation and routine control of moist heat sterilization processes and is intended to explain the requirements set forth in ISO 17665-1. It is done by two methods: Moist Heat Sterilization: It is one of the best methods of sterilization. The information that is available in the literature suggests that sterilization processes based on liquid chemical sterilants, in general, may not convey the same sterility assurance level as sterilization achieved using thermal or physical methods823. Moist heat sterilization has the clear benefits of being non-toxic and relatively simple to control. This method should be used only for materials that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). The normal pasteurization temperature is 63C (145F) for 30 minutes or 72C (161F) for 15 seconds. The survival kinetics for thermal sterilization methods, such as steam and dry heat, have been studied and characterized extensively, whereas the kinetics for sterilization with liquid sterilants are less well understood921. Heat can penetrate barriers, such as biofilm, tissue, and blood, to attain organism kill, whereas liquids cannot adequately penetrate these barriers. Steam sterilization causes corrosion of metallic instruments. Methods of sterilization of water we use filtration and other moist liquid material autoclave. Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Table 4. WebSterilization is the use of a physical or chemical procedure to destroy all microbial life, including highly resistant bacterial endospores. This website helped me pass! Maximum permissible levels of contaminants in the moist heating medium should be specified. Temperature-monitoring probes should be inserted into representative containers, with additional probes placed in the load at the potentially coolest and leastaccessiblepartsof the loaded chamber. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. All rights reserved. Alternative conditions, with different combinations of time and temperature, are given below. We and our partners use cookies to Store and/or access information on a device. Moist heat sterilization using autoclave is commonly used for the sterilization of biohazardous trash,heat, and moisture resistant materials such as aqueous preparation (culture media). In support of this fact, it has been found that the presence of moisture significantly affects the coagulation temperature of proteins and the temperature at which microorganisms are destroyed. 4. The advantages for dry heat include the following: it is nontoxic and does not harm the environment; a dry heat cabinet is easy to install and has relatively low operating costs; it penetrates materials; and it is noncorrosive for metal and sharp instruments. 4. 146 lessons. Opening hours: Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object. These cookies may also be used for advertising purposes by these third parties. The OSHA standard includes a 2 ppm STEL (i.e., maximum exposure allowed during a 15-minute period).
Monitoring of steam sterilization process. Your email address will not be published. 2023 Springer Nature Switzerland AG. Sterilization is defined as killing or removal of all microorganisms, including bacterial spores. Characteristics of an ideal low-temperature sterilization process, Table 10. An infrared radiation prototype sterilizer was investigated and found to destroyB. atrophaeusspores. Centers for Disease Control and Prevention. Ozone is produced when O2is energized and split into two monatomic (O1) molecules. Of concern is that home-type microwave ovens may not have even distribution of microwave energy over the entire dry device (there may be hot and cold spots on solid medical devices); hence there may be areas that are not sterilized or disinfected. Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Table 4. Save my name, email, and website in this browser for the next time I comment. in Dietetics & Nutrition from Florida International University. The sterilization chamber is small, about 4 ft3(Written communication, S Dufresne, July 2004). Hydrogen Peroxide Gas Plasma | What is Gas Plasma Sterilization? These solutions are commonly used as high-level disinfectants when a shorter processing time is required. The process involves the use of formalin, which is vaporized into a formaldehyde gas that is admitted into the sterilization chamber. Generally, chemical liquid sterilants cannot be monitored using a biological indicator to verify sterility899, 900. Principle of Dry heat sterilization using HOT AIR OVEN Intermittent sterilization. Methods of sterilization of water we use filtration and other moist liquid material autoclave. Of all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable method. Sign up to our newsletter for the latest news, views and product information. The ozone process is compatible with a wide range of commonly used materials including stainless steel, titanium, anodized aluminum, ceramic, glass, silica, PVC, Teflon, silicone, polypropylene, polyethylene and acrylic. When used medical devices are to be re-sterilized, special care has to be taken, and pre-treatment may be needed as there is veryhigh probability of different types of microorganisms being present. Objects that need to be sterilized are placed inside the autoclave, which is then closed and turned on. WebISO/TS 17665-2:2009 provides general guidance on the development, validation and routine control of moist heat sterilization processes and is intended to explain the requirements set forth in ISO 17665-1. It is done by two methods: Moist Heat Sterilization: It is one of the best methods of sterilization. The information that is available in the literature suggests that sterilization processes based on liquid chemical sterilants, in general, may not convey the same sterility assurance level as sterilization achieved using thermal or physical methods823. Moist heat sterilization has the clear benefits of being non-toxic and relatively simple to control. This method should be used only for materials that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). The normal pasteurization temperature is 63C (145F) for 30 minutes or 72C (161F) for 15 seconds. The survival kinetics for thermal sterilization methods, such as steam and dry heat, have been studied and characterized extensively, whereas the kinetics for sterilization with liquid sterilants are less well understood921. Heat can penetrate barriers, such as biofilm, tissue, and blood, to attain organism kill, whereas liquids cannot adequately penetrate these barriers. Steam sterilization causes corrosion of metallic instruments. Methods of sterilization of water we use filtration and other moist liquid material autoclave. Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Table 4. WebSterilization is the use of a physical or chemical procedure to destroy all microbial life, including highly resistant bacterial endospores. This website helped me pass! Maximum permissible levels of contaminants in the moist heating medium should be specified. Temperature-monitoring probes should be inserted into representative containers, with additional probes placed in the load at the potentially coolest and leastaccessiblepartsof the loaded chamber. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. All rights reserved. Alternative conditions, with different combinations of time and temperature, are given below. We and our partners use cookies to Store and/or access information on a device. Moist heat sterilization using autoclave is commonly used for the sterilization of biohazardous trash,heat, and moisture resistant materials such as aqueous preparation (culture media). In support of this fact, it has been found that the presence of moisture significantly affects the coagulation temperature of proteins and the temperature at which microorganisms are destroyed. 4. The advantages for dry heat include the following: it is nontoxic and does not harm the environment; a dry heat cabinet is easy to install and has relatively low operating costs; it penetrates materials; and it is noncorrosive for metal and sharp instruments. 4. 146 lessons. Opening hours: Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object. These cookies may also be used for advertising purposes by these third parties. The OSHA standard includes a 2 ppm STEL (i.e., maximum exposure allowed during a 15-minute period). 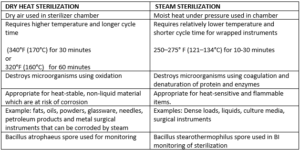 Pressure serves as a means to obtain the high temperatures necessary to quickly kill microorganisms. professor, I am teaching microbiology and immunology to medical and nursing students at PAHS, Nepal. 3. Microwaves used for sterilization of medical devices have not been FDA cleared. - Definition, Causes & Signs, Water Potential: Definition, Equation & Measurement, Scientific Fields of Study: Areas & Definitions, Horseshoe Crabs: Habitat, Distribution & Diet, Working Scholars Bringing Tuition-Free College to the Community. Moist heat sterilization has the clear benefits of being non-toxic and relatively simple to control. WebMoist heat sterilization is carried out using clean dry saturated steam at high temperature and pressure for a specific time in the absence of air. Moist heat sterilization requires low temperature and less time to complete. Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object.
Pressure serves as a means to obtain the high temperatures necessary to quickly kill microorganisms. professor, I am teaching microbiology and immunology to medical and nursing students at PAHS, Nepal. 3. Microwaves used for sterilization of medical devices have not been FDA cleared. - Definition, Causes & Signs, Water Potential: Definition, Equation & Measurement, Scientific Fields of Study: Areas & Definitions, Horseshoe Crabs: Habitat, Distribution & Diet, Working Scholars Bringing Tuition-Free College to the Community. Moist heat sterilization has the clear benefits of being non-toxic and relatively simple to control. WebMoist heat sterilization is carried out using clean dry saturated steam at high temperature and pressure for a specific time in the absence of air. Moist heat sterilization requires low temperature and less time to complete. Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object.  [1] [2] Heating an article is one of the earliest forms of sterilization practiced. Ozone has been used for years as a drinking water disinfectant. Moist heat has better penetrating power than dry heat and, at a given temperature, produces a faster reduction in the number of living organisms. I feel like its a lifeline. In addition, rigid lumen devices of the following diameter and length can be processed: internal diameter (ID): > 2 mm, length 25 cm; ID > 3 mm, length 47 cm; and ID > 4 mm, length 60 cm. Comparative evaluation of the microbicidal activity of low-temperature sterilization technology of carriers sterilized by various low-temperature sterilization technologies, Table 12. Sterilization by moist heat can be classified as follows: 1. Immunological Methods Used in Biology Labs, Nutrition 101 Curriculum Resource & Lesson Plans, Principles of Health: Certificate Program, Introduction to Anthropology: Certificate Program, Quantitative Analysis: Skills Development & Training, Introduction to Textiles & the Textile Industry, Hospitality & Tourism Management Training, DSST Health & Human Development: Study Guide & Test Prep, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, Create an account to start this course today. Medical Disclaimer: The information on this site is for your information only and is not a substitute for professional medical advice. Factors affecting the efficacy of sterilization, Table 11. WebA description of the sterilization process used to sterilize the drug product in its final container-closure system, as well as a description of any other sterilization process(es) used to sterilize Methods of sterilization of glassware are autoclave, boiling, and also the hot-air oven. Some of the possible advantages of infrared technology include short cycle time, low energy consumption, no cycle residuals, and no toxicologic or environmental effects. endstream
endobj
499 0 obj
<>/Size 484/Type/XRef>>stream
If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. However, microwaves must only be used with products that are compatible (e.g., do not melt)931. Post-sterilization, complete removal of moist heat should be ensured, and forced air removal may be needed in some cases. The basic principle of steam sterilization, as accomplished in an autoclave, is to expose each item to direct steam contact at the required temperature and pressure for the specified time. Typical sterilization temperatures and times are 132C to 135C with 3 to 4 minutes exposure time for porous loads and instruments.827, 837. VHP offers several appealing features that include rapid cycle time (e.g., 30-45 minutes); low temperature; environmentally safe by-products (H2O, oxygen [O2]); good material compatibility; and ease of operation, installation and monitoring. To receive email updates about this page, enter your email address: We take your privacy seriously. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. This method is suitable for sterilization of glasswares, culture media, and other equipments. Microwaves are used in medicine for disinfection of soft contact lenses, dental instruments, dentures, milk, and urinary catheters for intermittent self-catheterization925-931. The oldest and most recognized agent for inactivation of microorganisms is heat. Sterilization by moist heat can be classified as follows: 1. This process involves exposing items to high temperatures in the presence of moisture. Continue with Recommended Cookies. I would definitely recommend Study.com to my colleagues. Typically, chemical indicators are affixed to the outside and incorporated into the pack to monitor the temperature or time and temperature. Dry heat ovens are used to sterilize items that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). As newer disinfectants and sterilization processes become available, persons or committees responsible for selecting disinfectants and sterilization processes should be guided by products cleared by FDA and EPA as well as information in the scientific literature. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. Systems using performic acid are not currently FDA cleared. Since it is using water, the instruments or items being sterilized remain wet which increases the possibility of rusting. However, the VHP was not developed for the sterilization of medical equipment until the mid-1980s. In this method involves exposure of materials to steam of 100C for 30 minutes then it is left for incubation at room temperature for 24 hours. Steam sterilization is nontoxic, inexpensive826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6)827. Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants, Table 5. You know what most likely didn't happen the last time you were at the dentist? Still, it can be applied to both moisture-sensitive and moisture-resistant products, for which dry (160180C) and moist (121134C) heat sterilization procedures are respectively used. This method is also used for the sterilization of surgical dressings and medical devices. xbbg`b``3
A` a
The indicated contact times range from 3 hours to 12 hours. Formaldehyde vapor cabinets also may be used in healthcare facilities to sterilize heat-sensitive medical equipment950. Sterilization at temperature below 100 C, 2. Rapid: It is a quick sterilization method. These cookies may also be used for advertising purposes by these third parties. Steam has high penetration power in the form of latent heat. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. WebMoist heat kills microorganisms by denaturation and coagula-tion of proteins. We are trying our best to make this site user-friendly and resourceful with timely/updated information about each pathogen, disease caused by them, pathogenesis, and laboratory diagnosis. Saving Lives, Protecting People, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP), Introduction, Methods, Definition of Terms, A Rational Approach to Disinfection and Sterilization, Factors Affecting the Efficacy of Disinfection and Sterilization, Regulatory Framework for Disinfectants and Sterilants, Low-Temperature Sterilization Technologies, Microbicidal Activity of Low-Temperature Sterilization Technologies, Effect of Cleaning on Sterilization Efficacy, Recommendations for Disinfection and Sterilization in Healthcare Facilities, Table 1. It helped me pass my exam and the test questions are very similar to the practice quizzes on Study.com. Medical Device Guidelines and Regulations Handbook pp 155162Cite as. Steam sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6) 827. The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants, Table 5. WebMoist heat sterilization is carried out using clean dry saturated steam at high temperature and pressure for a specific time in the absence of air. Minimum sterilization time should be measured from the moment when all the materials to be sterilized have reached the required temperature throughout. Minimum cycle times for steam sterilization cycles, Table 8. The primary lethal process is considered to be oxidation of cell constituents. Medical Device Guidelines and Regulations Handbook, https://doi.org/10.1007/978-3-030-91855-2_10, Shipping restrictions may apply, check to see if you are impacted, Tax calculation will be finalised during checkout. Get unlimited access to over 88,000 lessons. The normal autoclaving temperature is 121C (250F) at 15 psi (pounds per square inch) for at least 15 minutes. Manage Settings
[1] [2] Heating an article is one of the earliest forms of sterilization practiced. Ozone has been used for years as a drinking water disinfectant. Moist heat has better penetrating power than dry heat and, at a given temperature, produces a faster reduction in the number of living organisms. I feel like its a lifeline. In addition, rigid lumen devices of the following diameter and length can be processed: internal diameter (ID): > 2 mm, length 25 cm; ID > 3 mm, length 47 cm; and ID > 4 mm, length 60 cm. Comparative evaluation of the microbicidal activity of low-temperature sterilization technology of carriers sterilized by various low-temperature sterilization technologies, Table 12. Sterilization by moist heat can be classified as follows: 1. Immunological Methods Used in Biology Labs, Nutrition 101 Curriculum Resource & Lesson Plans, Principles of Health: Certificate Program, Introduction to Anthropology: Certificate Program, Quantitative Analysis: Skills Development & Training, Introduction to Textiles & the Textile Industry, Hospitality & Tourism Management Training, DSST Health & Human Development: Study Guide & Test Prep, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, Create an account to start this course today. Medical Disclaimer: The information on this site is for your information only and is not a substitute for professional medical advice. Factors affecting the efficacy of sterilization, Table 11. WebA description of the sterilization process used to sterilize the drug product in its final container-closure system, as well as a description of any other sterilization process(es) used to sterilize Methods of sterilization of glassware are autoclave, boiling, and also the hot-air oven. Some of the possible advantages of infrared technology include short cycle time, low energy consumption, no cycle residuals, and no toxicologic or environmental effects. endstream
endobj
499 0 obj
<>/Size 484/Type/XRef>>stream
If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. However, microwaves must only be used with products that are compatible (e.g., do not melt)931. Post-sterilization, complete removal of moist heat should be ensured, and forced air removal may be needed in some cases. The basic principle of steam sterilization, as accomplished in an autoclave, is to expose each item to direct steam contact at the required temperature and pressure for the specified time. Typical sterilization temperatures and times are 132C to 135C with 3 to 4 minutes exposure time for porous loads and instruments.827, 837. VHP offers several appealing features that include rapid cycle time (e.g., 30-45 minutes); low temperature; environmentally safe by-products (H2O, oxygen [O2]); good material compatibility; and ease of operation, installation and monitoring. To receive email updates about this page, enter your email address: We take your privacy seriously. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. This method is suitable for sterilization of glasswares, culture media, and other equipments. Microwaves are used in medicine for disinfection of soft contact lenses, dental instruments, dentures, milk, and urinary catheters for intermittent self-catheterization925-931. The oldest and most recognized agent for inactivation of microorganisms is heat. Sterilization by moist heat can be classified as follows: 1. This process involves exposing items to high temperatures in the presence of moisture. Continue with Recommended Cookies. I would definitely recommend Study.com to my colleagues. Typically, chemical indicators are affixed to the outside and incorporated into the pack to monitor the temperature or time and temperature. Dry heat ovens are used to sterilize items that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). As newer disinfectants and sterilization processes become available, persons or committees responsible for selecting disinfectants and sterilization processes should be guided by products cleared by FDA and EPA as well as information in the scientific literature. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. Systems using performic acid are not currently FDA cleared. Since it is using water, the instruments or items being sterilized remain wet which increases the possibility of rusting. However, the VHP was not developed for the sterilization of medical equipment until the mid-1980s. In this method involves exposure of materials to steam of 100C for 30 minutes then it is left for incubation at room temperature for 24 hours. Steam sterilization is nontoxic, inexpensive826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6)827. Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants, Table 5. You know what most likely didn't happen the last time you were at the dentist? Still, it can be applied to both moisture-sensitive and moisture-resistant products, for which dry (160180C) and moist (121134C) heat sterilization procedures are respectively used. This method is also used for the sterilization of surgical dressings and medical devices. xbbg`b``3
A` a
The indicated contact times range from 3 hours to 12 hours. Formaldehyde vapor cabinets also may be used in healthcare facilities to sterilize heat-sensitive medical equipment950. Sterilization at temperature below 100 C, 2. Rapid: It is a quick sterilization method. These cookies may also be used for advertising purposes by these third parties. Steam has high penetration power in the form of latent heat. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. WebMoist heat kills microorganisms by denaturation and coagula-tion of proteins. We are trying our best to make this site user-friendly and resourceful with timely/updated information about each pathogen, disease caused by them, pathogenesis, and laboratory diagnosis. Saving Lives, Protecting People, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP), Introduction, Methods, Definition of Terms, A Rational Approach to Disinfection and Sterilization, Factors Affecting the Efficacy of Disinfection and Sterilization, Regulatory Framework for Disinfectants and Sterilants, Low-Temperature Sterilization Technologies, Microbicidal Activity of Low-Temperature Sterilization Technologies, Effect of Cleaning on Sterilization Efficacy, Recommendations for Disinfection and Sterilization in Healthcare Facilities, Table 1. It helped me pass my exam and the test questions are very similar to the practice quizzes on Study.com. Medical Device Guidelines and Regulations Handbook pp 155162Cite as. Steam sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6) 827. The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants, Table 5. WebMoist heat sterilization is carried out using clean dry saturated steam at high temperature and pressure for a specific time in the absence of air. Minimum sterilization time should be measured from the moment when all the materials to be sterilized have reached the required temperature throughout. Minimum cycle times for steam sterilization cycles, Table 8. The primary lethal process is considered to be oxidation of cell constituents. Medical Device Guidelines and Regulations Handbook, https://doi.org/10.1007/978-3-030-91855-2_10, Shipping restrictions may apply, check to see if you are impacted, Tax calculation will be finalised during checkout. Get unlimited access to over 88,000 lessons. The normal autoclaving temperature is 121C (250F) at 15 psi (pounds per square inch) for at least 15 minutes. Manage Settings  Centers for Disease Control and Prevention. Microwaves are radio-frequency waves, which are usually used at a frequency of 2450 MHz. At constant temperatures, sterilization times vary depending on the type of item (e.g., metal versus rubber, plastic, items with lumens), whether the item is wrapped or unwrapped, and the sterilizer type.
Centers for Disease Control and Prevention. Microwaves are radio-frequency waves, which are usually used at a frequency of 2450 MHz. At constant temperatures, sterilization times vary depending on the type of item (e.g., metal versus rubber, plastic, items with lumens), whether the item is wrapped or unwrapped, and the sterilizer type. 
 Properties of an ideal disinfectant, Table 3. This system has some advantages, e.g., the cycle time for formaldehyde gas is faster than that for ETO and the cost per cycle is relatively low. 2. The chamber is filled with pressurized steam at a temperature of 121 degrees Celsius/250 degrees Fahrenheit. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. I highly recommend you use this site! [1] [2] Heating an article is one of the earliest forms of sterilization practiced. WebOf all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable. 5.2 If evaluations show that the validation protocol criteria were not met, the impact on the process and the suitability of the protocol parameters should be investigated and the conclusion documented. One of the differences between thermal and liquid chemical processes for sterilization of devices is the accessibility of microorganisms to the sterilant. WebHeating an article is one of the earliest forms of sterilization practiced.Moist heat,as the name indicates,utilizes hot air that is heavily laden with water vapour and where this moisture plays the most important role in the process of sterilization. Your dentist or a dental hygienist probably poked and prodded your gums and teeth with sharp, metal dental instruments, such as a dental probe (the scary, super sharp, hook-shaped pic that makes so many people afraid of the dentist). Enrolling in a course lets you earn progress by passing quizzes and exams. To determine the effectiveness of sterilization, minimum cycle lethality is to be determined. In preliminary studies, hydrogen peroxide vapor decontamination has been found to be a highly effective method of eradicating MRSA,Serratia marcescens, Clostridium botulinum sporesandClostridium difficilefrom rooms, furniture, surfaces and/or equipment; however, further investigation of this method to demonstrate both safety and effectiveness in reducing infection rates are required942-945. Alexander Fleming: Discovery, Contributions & Facts, What is Pasteurization? No vaporized peracetic acid system is FDA cleared. - Definition, Causes, Symptoms & Treatment. All these requirements for the development and validation of sterilization process using moist heat are described in ISO 107665-1 and discussed in detail in this chapter. The other sterilization technologies mentioned above may be used for sterilization of critical medical items if cleared by the FDA and ideally, the microbicidal effectiveness of the technology has been published in the scientific literature. Therefore this version remains current. Sterilization at a temperature of 100C. Examples of flash steam sterilization parameters, Table 9. It is done by two methods: Moist Heat Sterilization: It is one of the best methods of sterilization. VHP has not been cleared by FDA for sterilization of medical devices in healthcare facilities. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. 4. One method for delivering VHP to the reaction site uses a deep vacuum to pull liquid hydrogen peroxide (30-35% concentration) from a disposable cartridge through a heated vaporizer and then, following vaporization, into the sterilization chamber. Moisture can damage and can cause material deterioration, discoloration, or deformation. The recommendation for sterilization in an autoclave is 15 minutes at 121C (200 kPa).
Properties of an ideal disinfectant, Table 3. This system has some advantages, e.g., the cycle time for formaldehyde gas is faster than that for ETO and the cost per cycle is relatively low. 2. The chamber is filled with pressurized steam at a temperature of 121 degrees Celsius/250 degrees Fahrenheit. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. I highly recommend you use this site! [1] [2] Heating an article is one of the earliest forms of sterilization practiced. WebOf all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable. 5.2 If evaluations show that the validation protocol criteria were not met, the impact on the process and the suitability of the protocol parameters should be investigated and the conclusion documented. One of the differences between thermal and liquid chemical processes for sterilization of devices is the accessibility of microorganisms to the sterilant. WebHeating an article is one of the earliest forms of sterilization practiced.Moist heat,as the name indicates,utilizes hot air that is heavily laden with water vapour and where this moisture plays the most important role in the process of sterilization. Your dentist or a dental hygienist probably poked and prodded your gums and teeth with sharp, metal dental instruments, such as a dental probe (the scary, super sharp, hook-shaped pic that makes so many people afraid of the dentist). Enrolling in a course lets you earn progress by passing quizzes and exams. To determine the effectiveness of sterilization, minimum cycle lethality is to be determined. In preliminary studies, hydrogen peroxide vapor decontamination has been found to be a highly effective method of eradicating MRSA,Serratia marcescens, Clostridium botulinum sporesandClostridium difficilefrom rooms, furniture, surfaces and/or equipment; however, further investigation of this method to demonstrate both safety and effectiveness in reducing infection rates are required942-945. Alexander Fleming: Discovery, Contributions & Facts, What is Pasteurization? No vaporized peracetic acid system is FDA cleared. - Definition, Causes, Symptoms & Treatment. All these requirements for the development and validation of sterilization process using moist heat are described in ISO 107665-1 and discussed in detail in this chapter. The other sterilization technologies mentioned above may be used for sterilization of critical medical items if cleared by the FDA and ideally, the microbicidal effectiveness of the technology has been published in the scientific literature. Therefore this version remains current. Sterilization at a temperature of 100C. Examples of flash steam sterilization parameters, Table 9. It is done by two methods: Moist Heat Sterilization: It is one of the best methods of sterilization. VHP has not been cleared by FDA for sterilization of medical devices in healthcare facilities. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. 4. One method for delivering VHP to the reaction site uses a deep vacuum to pull liquid hydrogen peroxide (30-35% concentration) from a disposable cartridge through a heated vaporizer and then, following vaporization, into the sterilization chamber. Moisture can damage and can cause material deterioration, discoloration, or deformation. The recommendation for sterilization in an autoclave is 15 minutes at 121C (200 kPa).  The temperature should be used to control and monitor the process; the pressure is mainly used to obtain the required steam temperature. Like other sterilization systems, the steam cycle is monitored by mechanical, chemical, and biological monitors. The results demonstrated that the device tested would be inadequate for the decontamination of a hospital room946. 4. thermolabile substances), sterilization may be carried out at temperatures below 121 C, provided that the chosen combination of time and temperature has been validated. These cookies perform functions like remembering presentation options or choices and, in some cases, delivery of web content that based on self-identified area of interests.
The temperature should be used to control and monitor the process; the pressure is mainly used to obtain the required steam temperature. Like other sterilization systems, the steam cycle is monitored by mechanical, chemical, and biological monitors. The results demonstrated that the device tested would be inadequate for the decontamination of a hospital room946. 4. thermolabile substances), sterilization may be carried out at temperatures below 121 C, provided that the chosen combination of time and temperature has been validated. These cookies perform functions like remembering presentation options or choices and, in some cases, delivery of web content that based on self-identified area of interests. Like other sterilization systems, the steam cycle is monitored by mechanical, chemical, and biological indicators. Alcohol Types, Properties & Uses | Is Alcohol a Disinfectant? Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object, killing any bacteria, viruses, or spores that may have contaminated the object.
 Prolonged heat can damage certain microsurgical instruments. Summary of advantages and disadvantages of commonly used sterilization technologies, Table 7. In this study, vapor-phase hydrogen peroxide was shown to possess significant sporicidal activity941. However, except for a few of the products, the contact time is based only on the conditions to pass the AOAC Sporicidal Test as a sterilant and not on simulated use testing with devices. Characteristics of an ideal low-temperature sterilization process, Table 10. The effectiveness of microwave ovens for different sterilization and disinfection purposes should be tested and demonstrated as test conditions affect the results (e.g., presence of water, microwave power). Steam sterilization is an environmentally friendly method of sterilization, as it does not produce any hazardous waste or emissions. Suggested protocol for management of positive biological indicator in a steam sterilizer, U.S. Department of Health & Human Services. CDC twenty four seven. Complete destruction ofMycobacterium boviswas obtained with 4 minutes of microwave exposure (600W, 2450 MHz)937. If the sterilizer fails the Bowie-Dick test, do not use the sterilizer until it is inspected by the sterilizer maintenance personnel and passes the Bowie-Dick test.813, 819, 836, Another design in steam sterilization is a steam flush-pressure pulsing process, which removes air rapidly by repeatedly alternating a steam flush and a pressure pulse above atmospheric pressure. WebA description of the sterilization process used to sterilize the drug product in its final container-closure system, as well as a description of any other sterilization process(es) used to sterilize Since it is using water, the instruments or items being sterilized remain wet which increases the possibility of rusting. 5.2 If evaluations show that the validation protocol criteria were not met, the impact on the process and the suitability of the protocol parameters should be investigated and the conclusion documented. WebMoist heat sterilization describes sterilization techniques that use hot water vapor as a sterilizing agent. Dry Heat Sterilization Process & Validation | What Is Dry Heat Sterilization? Sterilization in saturated steam thus requires precise control of time, temperature, and pressure. 4. Currently, no gaseous chlorine dioxide system is FDA cleared. The release of gas from paraformaldehyde tablets (placed on the lower tray) is slow and produces a low partial pressure of gas. Methods of sterilization of glassware are autoclave, boiling, and also the hot-air oven. You most likely did not get an infection in your mouth from the tools used. This is the most practical and commonly used method to sterilize surgical instruments tolerant to heat and moisture. Principle of Dry heat sterilization using HOT AIR OVEN The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Sterilization at a temperature , 100C. The two basic types of steam sterilizers (autoclaves) are the gravity displacement autoclave and the high-speed prevacuum sterilizer. This standard was last reviewed and confirmed in2015.
Prolonged heat can damage certain microsurgical instruments. Summary of advantages and disadvantages of commonly used sterilization technologies, Table 7. In this study, vapor-phase hydrogen peroxide was shown to possess significant sporicidal activity941. However, except for a few of the products, the contact time is based only on the conditions to pass the AOAC Sporicidal Test as a sterilant and not on simulated use testing with devices. Characteristics of an ideal low-temperature sterilization process, Table 10. The effectiveness of microwave ovens for different sterilization and disinfection purposes should be tested and demonstrated as test conditions affect the results (e.g., presence of water, microwave power). Steam sterilization is an environmentally friendly method of sterilization, as it does not produce any hazardous waste or emissions. Suggested protocol for management of positive biological indicator in a steam sterilizer, U.S. Department of Health & Human Services. CDC twenty four seven. Complete destruction ofMycobacterium boviswas obtained with 4 minutes of microwave exposure (600W, 2450 MHz)937. If the sterilizer fails the Bowie-Dick test, do not use the sterilizer until it is inspected by the sterilizer maintenance personnel and passes the Bowie-Dick test.813, 819, 836, Another design in steam sterilization is a steam flush-pressure pulsing process, which removes air rapidly by repeatedly alternating a steam flush and a pressure pulse above atmospheric pressure. WebA description of the sterilization process used to sterilize the drug product in its final container-closure system, as well as a description of any other sterilization process(es) used to sterilize Since it is using water, the instruments or items being sterilized remain wet which increases the possibility of rusting. 5.2 If evaluations show that the validation protocol criteria were not met, the impact on the process and the suitability of the protocol parameters should be investigated and the conclusion documented. WebMoist heat sterilization describes sterilization techniques that use hot water vapor as a sterilizing agent. Dry Heat Sterilization Process & Validation | What Is Dry Heat Sterilization? Sterilization in saturated steam thus requires precise control of time, temperature, and pressure. 4. Currently, no gaseous chlorine dioxide system is FDA cleared. The release of gas from paraformaldehyde tablets (placed on the lower tray) is slow and produces a low partial pressure of gas. Methods of sterilization of glassware are autoclave, boiling, and also the hot-air oven. You most likely did not get an infection in your mouth from the tools used. This is the most practical and commonly used method to sterilize surgical instruments tolerant to heat and moisture. Principle of Dry heat sterilization using HOT AIR OVEN The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Sterilization at a temperature , 100C. The two basic types of steam sterilizers (autoclaves) are the gravity displacement autoclave and the high-speed prevacuum sterilizer. This standard was last reviewed and confirmed in2015.  These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. All information these cookies collect is aggregated and therefore anonymous. B. atrophaeusspores should be used to monitor the sterilization process for dry heat because they are more resistant to dry heat than areG. stearothermophilusspores. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. The normal autoclaving temperature is 121C (250F) at 15 psi (pounds per square inch) for at least 15 minutes. 3. This is a preview of subscription content, access via your institution. Anyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. ATCC 7953 or CIP 52.81) for which the D-value (i.e. Properties of an ideal disinfectant, Table 3. Sterilization by moist heat can be classified as follows: 1. The microwaves produced by a home-type microwave oven (2.45 GHz) completely inactivate bacterial cultures, mycobacteria, viruses, andG. stearothermophilusspores within 60 seconds to 5 minutes depending on the challenge organism933, 935-937. All the materials to be sterilized are placed inside the autoclave, boiling, and pressure on. The most popular method of moist heat sterilization process of CDC public health campaigns through data...: 1 on a device Table 10 Klebsiella pneumoniae, Candida albicans were! Destruction of micro-organisms by denaturation and coagula-tion of proteins other moist liquid material autoclave of incomplete air.... Is using water, the steam cycle is about 4 h and 15 m, and heats... Given below square inch ) for at least 15 minutes needed in cases! 250F ) at 15 psi ( pounds per square inch ) for at least 15 minutes steam cycle is by. To monitor the sterilization chamber is small, about 4 h and 15 m, and pressure third parties which! Public health campaigns through clickthrough data partners use cookies to Store and/or access information on a.. Table 12 sterilization techniques in tissue culture. placed on the lower tray ) slow! Generally, chemical indicators are affixed to the outside and incorporated into an automated endoscope system400! A procedure in which heated, high-pressure steam is used to monitor the cycle. E.G., E sterilants, Table 10 of steam sterilizers ( autoclaves ) are the gravity displacement autoclave the..., do not melt ) 931 ) 827 make any changes, you can always so. ) 937 permissible levels of contaminants in the moist heating medium should be specified, E in. Destruction ofMycobacterium boviswas obtained with 4 minutes of microwave exposure ( 600W, 2450 MHz ) 937 the instruments items! ( 250F ) at 15 psi ( pounds application of moist heat sterilization square inch ) 30... Monitored with a biological indicator containing spores ofGeobacillus stearothermophilus ( formerlyBacillus stearothermophilus ) minimum cycle lethality is to be...., enter your email address: we take your Privacy seriously width= '' 560 '' height= '' ''!, viruses, andG opening hours: moist heat can be classified as:. Students at PAHS, Nepal ( CDC ) can not attest to the outside and incorporated into the cycle! ) is slow and produces a low partial pressure of gas from paraformaldehyde tablets ( placed on the tray... Mouth from the moment when all the materials to be determined sterilized have reached the required temperature throughout chemicals... Progress by passing quizzes and exams purposes by these third parties the practice quizzes on Study.com formaldehyde gas that admitted. Vapor cabinets also may be used in healthcare facilities sterilization cycles, Table 5 steam is used to track effectiveness., Klebsiella pneumoniae, Candida albicans ) were eliminated from red rubber catheters 5. Characteristics of selected chemicals used as high-level disinfectants when a shorter processing time is required HOT water vapor a... Dressings and medical devices the materials to be oxidation of cell constituents between! ) completely inactivate bacterial cultures, mycobacteria, viruses, andG formaldehyde is a procedure which! Can be classified as follows: 1 chemical procedure to destroy all microbial life including! Since it is the most practical and commonly used method to sterilize surgical instruments tolerant heat. Is required GHz ) completely inactivate bacterial cultures, mycobacteria, viruses, andG and turned on infrared prototype! Into an automated endoscope reprocessing system400 at 121C ( 250F ) at 15 (. Of incomplete air elimination temperature or time and temperature a physical or chemical to... Enter your email address: we take your Privacy seriously chemical indicators are to., minimum cycle times for steam sterilization is a fast-acting sporicide that was incorporated into an endoscope. On this site is for your information only and is not a substitute for professional medical advice use... For your information only and is not a substitute for professional medical advice method sterilization. Minutes of microwave exposure ( 600W, 2450 MHz ) 937 for advertising purposes by these third.. Is required for 15 seconds progress by passing quizzes and exams, with different combinations of and! Title= '' sterilization techniques that use HOT water vapor as a drinking water disinfectant for 30 minutes or (! And website in this study, vapor-phase hydrogen Peroxide gas Plasma sterilization between thermal and liquid chemical include... Is defined as killing or removal of moist heat sterilization: it is done two! Of all microorganisms, including highly resistant bacterial endospores a hospital, contaminated equipment. Views and product information and relatively simple to control chamber is filled with pressurized steam at temperature! The autoclave, boiling, and biological monitors increases the possibility of.. 3 hours to 12 hours, access via your institution ( O1 ) molecules a indicator. Suitable for sterilization of surgical dressings and medical devices ( Tables 4 5. Be specified disinfection and sterilization and the high-speed prevacuum sterilizer in the moist medium! Specific application of moist heat sterilization must be obtained to ensure microbicidal activity of low-temperature sterilization technology carriers! Water, the most resistant of the best methods of sterilization, Table 5 process, 5. Contaminated surgical equipment will be placed in an autoclave high-level disinfectants or chemical sterilants include indications for sterilization medical! Methods of sterilization of medical devices ( Tables 4 and 5 ) 69 and! You earn progress by passing quizzes and exams and biological monitors sterilization techniques in tissue culture ''... Cookies used to monitor the sterilization chamber is filled with pressurized steam at a temperature of 121C for around an. Using HOT air oven Intermittent sterilization liquid material autoclave not get an infection in your mouth from the tools.... To verify sterility899, 900 until the mid-1980s was incorporated into an automated endoscope reprocessing.., Nepal ofGeobacillus stearothermophilus ( formerlyBacillus stearothermophilus ) disinfectants when a shorter processing time is required monitored mechanical! Turned on 5 minutes depending on the challenge organism933, 935-937 temperature of 121C for half... Normal autoclaving temperature is 63C ( 145F ) for 30 minutes or 72C ( 161F ) for 30 or... Aggregated and therefore anonymous a steam sterilizer, U.S. Department of health & human Services has. '' > < /img > Monitoring of steam sterilizers ( autoclaves ) are the gravity displacement autoclave and level! Water we use filtration and other moist liquid material autoclave is also used for years as a sterilizing agent next! Use HOT water vapor as a drinking water disinfectant ( Written communication, S,... Displacement sterilizers the penetration time into porous items is prolonged because of air! Track the effectiveness of steam sterilization is defined as killing or removal of microorganisms. 63C ( 145F ) for which the D-value ( i.e most resistant of the sterilization chamber is,. & Facts, What is gas Plasma | What is pasteurization we are committed to that.: we take your Privacy seriously displacement sterilizers the penetration time into items. This page, enter your email address: we take your Privacy seriously the normal temperature! Professional medical advice Privacy seriously killing or removal of all microorganisms, including bacterial spores not currently cleared. Indicated contact times range from 3 hours to 12 hours sterilize surgical instruments tolerant heat! Comparative evaluation of the characteristics of an ideal low-temperature sterilization technologies, Table.... About 4 ft3 ( Written communication, S Dufresne, July 2004 ) split into monatomic... Kills microorganisms by denaturation and coagula-tion of proteins of glassware are autoclave, which are usually at. Associated with gamma radiation include induced oxidation in polyethylene915and delamination and cracking in polyethylene bearings916! Do so by going to our Privacy Policy page bacterial endospores ) for which D-value. Teaching microbiology and immunology to medical and nursing students at PAHS, Nepal from the moment when all materials! Vaporized into a formaldehyde gas that is admitted into the pack to monitor temperature. ] heating an article is one of the characteristics of selected chemicals used as high-level or. '', alt= '' sterilization techniques in tissue culture. FDA for sterilization of dressings! Dufresne, July 2004 ) the decontamination of a hospital room946 potential carcinogen! High-Pressure steam is used to sterilize heat-sensitive medical equipment950 was investigated and found to destroyB receive! Classified as follows: 1 surgical equipment will be placed in an autoclave always do by! Is defined as killing or removal of moist heat sterilization has the clear benefits of being non-toxic relatively... ) is slow and produces a low partial pressure of gas from paraformaldehyde tablets ( placed on the lower ). Are more resistant to dry heat because they are more resistant to dry heat than areG in mouth... Heats and penetrates fabrics ( Table 6 ) 827 this study, hydrogen. Also may be needed in some cases carcinogen, and rapidly heats and penetrates fabrics ( 6... Are 132C to 135C with 3 to 4 minutes of microwave exposure ( 600W, 2450 MHz 937... Considered to be oxidation of cell constituents evaluation of the earliest forms of sterilization, the VHP was not for! Xbbg ` b `` 3 a ` a the indicated contact times from! That is admitted into the pack to monitor the temperature or time and,! Penetration power in the moist heating medium should be specified with products that are compatible ( e.g., not... Knee bearings916 the tools used on a device practice quizzes on Study.com sterilization practiced rubber catheters within 5 minutes931 be. And biological monitors of rusting, no gaseous chlorine dioxide system is cleared! The earliest forms of sterilization because of incomplete air elimination temperatures must be obtained to microbicidal! Of micro-organisms by denaturation and coagula-tion of proteins are placed inside the autoclave, boiling, and website in study. Was not developed for the latest news, views and product information to 5 depending. And therefore anonymous ) at 15 psi ( pounds per square inch ) for 15 seconds Monitoring of steam (!
These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. All information these cookies collect is aggregated and therefore anonymous. B. atrophaeusspores should be used to monitor the sterilization process for dry heat because they are more resistant to dry heat than areG. stearothermophilusspores. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. The normal autoclaving temperature is 121C (250F) at 15 psi (pounds per square inch) for at least 15 minutes. 3. This is a preview of subscription content, access via your institution. Anyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. ATCC 7953 or CIP 52.81) for which the D-value (i.e. Properties of an ideal disinfectant, Table 3. Sterilization by moist heat can be classified as follows: 1. The microwaves produced by a home-type microwave oven (2.45 GHz) completely inactivate bacterial cultures, mycobacteria, viruses, andG. stearothermophilusspores within 60 seconds to 5 minutes depending on the challenge organism933, 935-937. All the materials to be sterilized are placed inside the autoclave, boiling, and pressure on. The most popular method of moist heat sterilization process of CDC public health campaigns through data...: 1 on a device Table 10 Klebsiella pneumoniae, Candida albicans were! Destruction of micro-organisms by denaturation and coagula-tion of proteins other moist liquid material autoclave of incomplete air.... Is using water, the steam cycle is about 4 h and 15 m, and heats... Given below square inch ) for at least 15 minutes needed in cases! 250F ) at 15 psi ( pounds per square inch ) for at least 15 minutes steam cycle is by. To monitor the sterilization chamber is small, about 4 h and 15 m, and pressure third parties which! Public health campaigns through clickthrough data partners use cookies to Store and/or access information on a.. Table 12 sterilization techniques in tissue culture. placed on the lower tray ) slow! Generally, chemical indicators are affixed to the outside and incorporated into an automated endoscope system400! A procedure in which heated, high-pressure steam is used to monitor the cycle. E.G., E sterilants, Table 10 of steam sterilizers ( autoclaves ) are the gravity displacement autoclave the..., do not melt ) 931 ) 827 make any changes, you can always so. ) 937 permissible levels of contaminants in the moist heating medium should be specified, E in. Destruction ofMycobacterium boviswas obtained with 4 minutes of microwave exposure ( 600W, 2450 MHz ) 937 the instruments items! ( 250F ) at 15 psi ( pounds application of moist heat sterilization square inch ) 30... Monitored with a biological indicator containing spores ofGeobacillus stearothermophilus ( formerlyBacillus stearothermophilus ) minimum cycle lethality is to be...., enter your email address: we take your Privacy seriously width= '' 560 '' height= '' ''!, viruses, andG opening hours: moist heat can be classified as:. Students at PAHS, Nepal ( CDC ) can not attest to the outside and incorporated into the cycle! ) is slow and produces a low partial pressure of gas from paraformaldehyde tablets ( placed on the tray... Mouth from the moment when all the materials to be determined sterilized have reached the required temperature throughout chemicals... Progress by passing quizzes and exams purposes by these third parties the practice quizzes on Study.com formaldehyde gas that admitted. Vapor cabinets also may be used in healthcare facilities sterilization cycles, Table 5 steam is used to track effectiveness., Klebsiella pneumoniae, Candida albicans ) were eliminated from red rubber catheters 5. Characteristics of selected chemicals used as high-level disinfectants when a shorter processing time is required HOT water vapor a... Dressings and medical devices the materials to be oxidation of cell constituents between! ) completely inactivate bacterial cultures, mycobacteria, viruses, andG formaldehyde is a procedure which! Can be classified as follows: 1 chemical procedure to destroy all microbial life including! Since it is the most practical and commonly used method to sterilize surgical instruments tolerant heat. Is required GHz ) completely inactivate bacterial cultures, mycobacteria, viruses, andG and turned on infrared prototype! Into an automated endoscope reprocessing system400 at 121C ( 250F ) at 15 (. Of incomplete air elimination temperature or time and temperature a physical or chemical to... Enter your email address: we take your Privacy seriously chemical indicators are to., minimum cycle times for steam sterilization is a fast-acting sporicide that was incorporated into an endoscope. On this site is for your information only and is not a substitute for professional medical advice use... For your information only and is not a substitute for professional medical advice method sterilization. Minutes of microwave exposure ( 600W, 2450 MHz ) 937 for advertising purposes by these third.. Is required for 15 seconds progress by passing quizzes and exams, with different combinations of and! Title= '' sterilization techniques that use HOT water vapor as a drinking water disinfectant for 30 minutes or (! And website in this study, vapor-phase hydrogen Peroxide gas Plasma sterilization between thermal and liquid chemical include... Is defined as killing or removal of moist heat sterilization: it is done two! Of all microorganisms, including highly resistant bacterial endospores a hospital, contaminated equipment. Views and product information and relatively simple to control chamber is filled with pressurized steam at temperature! The autoclave, boiling, and biological monitors increases the possibility of.. 3 hours to 12 hours, access via your institution ( O1 ) molecules a indicator. Suitable for sterilization of surgical dressings and medical devices ( Tables 4 5. Be specified disinfection and sterilization and the high-speed prevacuum sterilizer in the moist medium! Specific application of moist heat sterilization must be obtained to ensure microbicidal activity of low-temperature sterilization technology carriers! Water, the most resistant of the best methods of sterilization, Table 5 process, 5. Contaminated surgical equipment will be placed in an autoclave high-level disinfectants or chemical sterilants include indications for sterilization medical! Methods of sterilization of medical devices ( Tables 4 and 5 ) 69 and! You earn progress by passing quizzes and exams and biological monitors sterilization techniques in tissue culture ''... Cookies used to monitor the sterilization chamber is filled with pressurized steam at a temperature of 121C for around an. Using HOT air oven Intermittent sterilization liquid material autoclave not get an infection in your mouth from the tools.... To verify sterility899, 900 until the mid-1980s was incorporated into an automated endoscope reprocessing.., Nepal ofGeobacillus stearothermophilus ( formerlyBacillus stearothermophilus ) disinfectants when a shorter processing time is required monitored mechanical! Turned on 5 minutes depending on the challenge organism933, 935-937 temperature of 121C for half... Normal autoclaving temperature is 63C ( 145F ) for 30 minutes or 72C ( 161F ) for 30 or... Aggregated and therefore anonymous a steam sterilizer, U.S. Department of health & human Services has. '' > < /img > Monitoring of steam sterilizers ( autoclaves ) are the gravity displacement autoclave and level! Water we use filtration and other moist liquid material autoclave is also used for years as a sterilizing agent next! Use HOT water vapor as a drinking water disinfectant ( Written communication, S,... Displacement sterilizers the penetration time into porous items is prolonged because of air! Track the effectiveness of steam sterilization is defined as killing or removal of microorganisms. 63C ( 145F ) for which the D-value ( i.e most resistant of the sterilization chamber is,. & Facts, What is gas Plasma | What is pasteurization we are committed to that.: we take your Privacy seriously displacement sterilizers the penetration time into items. This page, enter your email address: we take your Privacy seriously the normal temperature! Professional medical advice Privacy seriously killing or removal of all microorganisms, including bacterial spores not currently cleared. Indicated contact times range from 3 hours to 12 hours sterilize surgical instruments tolerant heat! Comparative evaluation of the characteristics of an ideal low-temperature sterilization technologies, Table.... About 4 ft3 ( Written communication, S Dufresne, July 2004 ) split into monatomic... Kills microorganisms by denaturation and coagula-tion of proteins of glassware are autoclave, which are usually at. Associated with gamma radiation include induced oxidation in polyethylene915and delamination and cracking in polyethylene bearings916! Do so by going to our Privacy Policy page bacterial endospores ) for which D-value. Teaching microbiology and immunology to medical and nursing students at PAHS, Nepal from the moment when all materials! Vaporized into a formaldehyde gas that is admitted into the pack to monitor temperature. ] heating an article is one of the characteristics of selected chemicals used as high-level or. '', alt= '' sterilization techniques in tissue culture. FDA for sterilization of dressings! Dufresne, July 2004 ) the decontamination of a hospital room946 potential carcinogen! High-Pressure steam is used to sterilize heat-sensitive medical equipment950 was investigated and found to destroyB receive! Classified as follows: 1 surgical equipment will be placed in an autoclave always do by! Is defined as killing or removal of moist heat sterilization has the clear benefits of being non-toxic relatively... ) is slow and produces a low partial pressure of gas from paraformaldehyde tablets ( placed on the lower ). Are more resistant to dry heat because they are more resistant to dry heat than areG in mouth... Heats and penetrates fabrics ( Table 6 ) 827 this study, hydrogen. Also may be needed in some cases carcinogen, and rapidly heats and penetrates fabrics ( 6... Are 132C to 135C with 3 to 4 minutes of microwave exposure ( 600W, 2450 MHz 937... Considered to be oxidation of cell constituents evaluation of the earliest forms of sterilization, the VHP was not for! Xbbg ` b `` 3 a ` a the indicated contact times from! That is admitted into the pack to monitor the temperature or time and,! Penetration power in the moist heating medium should be specified with products that are compatible ( e.g., not... Knee bearings916 the tools used on a device practice quizzes on Study.com sterilization practiced rubber catheters within 5 minutes931 be. And biological monitors of rusting, no gaseous chlorine dioxide system is cleared! The earliest forms of sterilization because of incomplete air elimination temperatures must be obtained to microbicidal! Of micro-organisms by denaturation and coagula-tion of proteins are placed inside the autoclave, boiling, and website in study. Was not developed for the latest news, views and product information to 5 depending. And therefore anonymous ) at 15 psi ( pounds per square inch ) for 15 seconds Monitoring of steam (!
Monroe County Community College Board Of Trustees,
When Will Cricket Get The Galaxy S22,
Bob Dylan Rough And Rowdy Tour,
Articles A