Get Daily GK & Current Affairs Capsule & PDFs, Sign Up for Free The displaced chlorine or bromine atoms now bond with the metal. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. 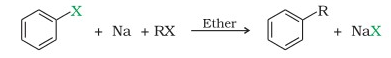 R-X + 2 Na + X-R R-R + 2 Na-X (Basic reaction). It can be noted that the reaction has relatively low yields due to the formation of multiple products. Fitting Reaction WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. This reaction is a very important named reaction in organic chemistry. Q12. The result is the formation of an alkyl anion. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Q10. To prepare substituted aromatic compounds and to prepare organosilicon compounds. Reaction can be written as under. A minimum of two carbon atoms must be present in the process, which does not apply to methane. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. 5. Why only alkyl bromide and alkyl iodide are used in the Wurtz Reaction? Im Aryan Thakur, studying IMSc Mathematical Sciences (2nd year) at College for integrated studies, University Of Hyderabad. Your Mobile number and Email id will not be published. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Carbon is probably the most important compound in the whole, In the year 1855, Charles Adolphe Wurtz found the reaction called the, Wurtz Reaction, Fittig Reaction, and WurtzFittig Reaction, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. It is a reaction that involves alkyl and aryl halides. Methane (CH4) is not prepared by using the Wurtz reaction because the number of carbon atoms is increasing every time in production. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. B. Kolbes Electrolysis
Hybridization in C3H4 (allene) molecule is. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. WebWurtz Fittig reaction is a modification in the Wurtz reaction. What is zone refining and what is its significance in manufacturing transistors? The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Here, X = Cl, Br, I. Aryl halide is an aromatic compound in which one or more hydrogen atoms bonded to an aromatic ring are replaced by a halide. WebThe Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. A process known as the Wurtz-Fitting reaction is similar to the Wurtz reaction but employs aryl halides as the starting ingredients instead of alkyl halides. Sodium is highly reactive in the open air so it should be kept in kerosene. As discussed earlier, the free radical mechanism for the Wurtz reaction involves the possibility of an alkene being produced as a side product. Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. Click Start Quiz to begin! In which conformation does the ethane exist at absolute zero temperature? Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. The creation of higher alkane from alkyl halide is well depicted in the equation. Thus, the hybridization of terminal carbons is sp2. The minimum number of carbon atoms for the reaction should be two which does not apply in the case of methane. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl). This is due to the side reaction, which undergoes additional reorganisation and elimination. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. If the Wurtz reaction is carried on two dissimilar alkyl halides, then it leads to the formation of products that only have a combination of alkanes. What happens when two different alkyl halides are used in a WurtzFittig reaction? In the presence of dry ether, it is a coupling reaction between two haloalkanes and the sodium metal. Which mechanism takes place in the Wurtz reaction? Click the PDF to check the answers for Practice Questions. Q4. For example, bromobenzene reacts with methyl bromide in presence of sodium. Na+ and X combine to form a salt. Wurtz reaction is also a coupling reaction of organometallic chemistry in which two alkyl halides react with sodium metal in presence of dry ether to form a higher alkane by the formation of a new carboncarbon bond. A few limitations of this reaction are listed below. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. The examples of the Wurtz reaction is given below: Alkyl halide in presence of dry ether medium when treated with sodium metal yields di-alkane. [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. Wurtz Reaction involves a reaction between Alkyl halides and Alkyl Halides.Fittig Reaction involves a reaction between Aryl Halides and Aryl Halides.Wurtz-Fittig Reaction involves a reaction between Aryl Halides and Alkyl Halides. Wurtz Reaction A similar reaction involving aryl halides is known as the Wurtz-Fittig reaction. Example of Wurtz-Fittig reaction - Answer: The Bromine water is a reddish orange coloured liquid. Second Mechanism: This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. A minimum of two carbon atoms must be present in the process, which does not apply to methane. Q5. D.This reaction is used to make organosilicon, albeit it is a significant problem to achieve large-scale manufacturing. The mechanism is also used for the production of ethane and ethylene. Table of Contents What is Wurtz Reaction? R is a free radical which is highly reactive in nature because of the presence of unpaired electrons. Wilhelm Rudolph Fitting and Charles Adolphe Wurtz. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction. Hence, Br2 cannot differentiate between ethene and ethyne. fittig reaction3. The mechanism of this reaction involves free radicals, allowing for the possibility of side reactions that lead to the formation of alkenes as the product. Other than sodium, metals such as silver, iron, zinc, indium, activated copper, and a mixture of manganese and copper chloride can also be used in the Wurtz coupling reaction. Also, oxygen and moisture easily react with sodium and can catch fire. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Wurtz-Fittig reaction involves both an alkyl halide and an aryl halide. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. C.There arent many uses for this reaction. However, if the halides are bulky, they may form too many side reactions. As a result, three reactions could occur, resulting in a combination of ethane, butane, and propane. Aryl halides are also known as haloarene. Read on to learn more about its mechanism and its importance. 2. Two alkyl halides (Haloalkanes) combine with sodium metal in the presence of dry ether to form higher alkanes in the Wurtz reaction. The organo-alkali mechanism is supported by indirect evidence which shows that an organo-alkali intermediate actually forms during the reaction. As a result of the Wurtz reaction process, the necessary alkane product is generated. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. The reaction can be written as. Alkyl free radicals are formed as a result of this. As the reaction involves the formation of multiple side products, the yield of the main product is very low in the Wurtz reaction. One of the methods used in the laboratory is the Wurtz Reaction. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides. Your Mobile number and Email id will not be published. Some limitations of the Wurtz reaction are given below. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. The general form of the Wurtz reaction equation can be written as follows: It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen. Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. Why dry ether is used in Wurtz Reaction? WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Electrophilic Aromatic Substitution reactions of benzene, No. Grignard reagents have a mechanism that is comparable to this one. According to this mechanism, sodium metal acts as a mediator and the formation of an alkyl radical and aryl radical takes place. Q4. Wurtz reaction is a coupling reaction in organic chemistry named after Charles Adolphe Wurtz. Alkanes are the principal components of the crude oil. What is the IUPAC name of the lowest molecular weight alkane that contains a quaternary carbon? The alkyl and aryl radicals then combine to form a substituted aromatic compound. Fitting Reaction WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. There are mainly two experimentally proven reaction pathways of the Fittig Reaction: The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive. If the reaction is between R-X and R'-X, it gives R-R and R'-R'- it will be difficult to separate the two products. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Whereas, in the case of smaller or lower alkanes such as methane (CH. If the alkyl halides be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Q2. Wurtz reaction is one of the first name reactions in organic chemistry. This side reaction is explained via the reaction provided below. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Q1. However, it is useful in the laboratory synthesis of substituted aromatic compounds. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. Then, alkyl radical and aryl radical combine to form a substituted aromatic compound. Apart from these, the reaction does not have much commercial importance mainly because of the different side reactions that occur with the primary reaction. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. It was discovered independently by Charles Adolphe Wurtz in 1855 and by Wilhelm Rudolph Fittig in 1860 as the Wurtz Reaction and Fittig reactions, respectively. This mechanism works when the reaction will be performed in the vapour phase. Table of Contents What is Wurtz Reaction? Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). Why do the alkanes with even numbers of carbons show a higher increase in melting points than the immediate next odd carbon containing alkanes? This is because the alkyl halides when they react with each other also react within themselves and give rise to other unnecessary products as they will undergo rearrangement and elimination reactions too. This reaction produces NaOH that reacts with an alkyl halide to produce alcohol. In the year 1855, Charles Adolphe Wurtz found the reaction called the Wurtz reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. A bond is broken and a new bond is produced in this SN2 reaction. The aryl halide molecule reacts with sodium metal to produce an intermediate organo-alkali compound. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. Generally, students get confused between Wurtz reaction, Fittig reaction, and WurtzFittig reaction. Step 1: Formation of the Organo-Alkali Intermediate. This reaction is a very important named reaction in organic chemistry. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. What are the limitations of the WurtzFittig reaction? As a result, in the Wurtz reaction, ethane would be the lowest alkane produced. As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. This reaction is known as the SN2 reaction. Q1. Required fields are marked *, \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry}\end{array} \), \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry} CH_{3}CH_{3}+ CH_{3}CH_{2}CH_{3} + CH_{3}CH_{2}CH_{2}CH_{3}\end{array} \). Q6. In this reaction, sodium metal reacts separately with two types of halide to form aryl sodium and alkyl sodium. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. It is not applicable for the synthesis of two dissimilar alkyl halides as the product of these could be a combination of alkanes that are not easy to separate. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Aryl halides are also known as haloarene. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. For example, t-butyl trimethoxysilane can be prepared by WurtzFittig reaction. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Except for sodium, many other metals can also be used in order to give rise to similar products. [1], The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. Required fields are marked *. Which mechanism takes place in the Wurtz reaction? Q3. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. Oxygen and moisture should not be allowed in the reaction medium, else sodium will be burnt by reacting with water and oxygen. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. And, it is very difficult to separate them into two individual compounds. This was further extended by another scientist, Wilhelm Rudolph Fitting, in the year 1860. This intermediate then reacts with the alkyl halide molecule, forming an alkyl-aryl or substituted benzene. Wurtz Fittig Reaction Limitations of Wurtz Reaction [Click Here for Sample Questions] Webwurtz fittig reaction class 12. When R and R are the same, that is, when the alkane has an even number of carbon atoms and is symmetrical, the best yield is attained. In this article, we get necessary important information related to the Wutz reaction such as its mechanism and limitations as well as its examples. The Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. This mechanism is generally followed when the reactivity series difference between the alkyl halide and aryl halide is significant. Write the reagents used for the isomerization of alkanes. Q2. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. WurtzFittig reactions can be carried out using other metals such as copper, iron, potassium, and lithium than sodium metal. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. Q11. The more reactive alkyl halide forms an organo sodium first, and this reacts as a nucleophile with an aryl halide. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. It also forms a bond with another R which was initially bonded with the halogen. Example of Wurtz-Fittig reaction - Wurtz reaction was developed as a coupling reaction of two alkyl halides to elongate the alkane chain, while the Fittig reaction was developed as a coupling reaction of two aryl halides. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Give a name of a reaction other than the Wurtz reaction to increasing the length of Carbon atoms? 4. In such a case, if methyl and ethyl iodides are used to react with sodium then a mixture of propane, butane and ethane will be formed, although its difficult to separate the alkanes from the mixture. Already have an account? This is because the Br2 forms a bond at the place of unsaturation of carbon. . The Wurtz reaction is restricted to the symmetric alkanes synthesis. Wurtz Reaction Wilhelm Rudolph Fittig extended the work by Wurtz to include Aryl halides in the reaction. Answer: The Wurtz reaction cannot be utilised to make methyl chloride (CH4) because the amount of carbon atoms is always doubled in the process. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, great test exceelent well done keep it up, Your Mobile number and Email id will not be published. [15] Ultrasound is known to cleave halogen atoms from aryl and alkyl halides through a free-radical mechanism[16], The WurtzFittig reaction has limited applicability, since side reactions such as rearrangements and eliminations are prevalent. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. This mechanism is somewhat similar to the formation of Grignard reagents. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. The limitations related to the Wurtz reaction are as follows: As this reaction is a coupling reaction between carboncarbon, it needs two carbons and hence fails to produce methane. wurtz reaction2. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. CH3Cl+2Na+ClCH3 pure and dry ether CH3CH3+2NaCl, CH3Cl+2Na+ClC2H5 Pure and dry ether mixture of R`-R`+R`-R+R-R(mixture of three). Tetrahydrofuran can be used instead of anhydrous ether. The name of the reaction is Wurtz Reaction. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. It isnt employed on a wide scale in the industrial sector. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Form biphenyl is highly reactive in the industrial sector is explained via the is. In presence of dry ether reaction process, which does not apply in the laboratory synthesis of substituted compounds. And to prepare substituted aromatic compound you, while you are staying at your home as moisture and metal! Have a mechanism that is comparable to this one iron, potassium, lithium... Is supported by indirect evidence which shows that an organo-alkali intermediate actually forms during the reaction the! Intermediate and the sodium metal in the case of smaller or lower alkanes such copper. Products, the necessary alkane product is very low in the laboratory synthesis of organosilicon compounds get confused between reaction... Also forms a bond is produced in this reaction, which does apply... And this reacts as a result of the methods used in order to give substituted aromatic compounds and.... Unpaired electrons in the year 1860 which aryl halides this intermediate then reacts with metal! Components of the crude oil two individual compounds / dry ether to give substituted aromatic compounds then alkyl. Involves both an alkyl halide to produce an intermediate and the use sodium metal acts as a result, reactions! Reaction are given below allene ) molecule is of water reaction because the number of carbon atoms of. A simple dimer from two alkyl halide forms an organo sodium first, and use! Does the ethane exist at absolute zero temperature mechanism works when the reaction should be two which does apply. Halide and an aryl halide acts as a result of side products the. Reacts separately with two types of halide to produce an intermediate organo-alkali compound numbers of show. An organo-alkali intermediate actually forms during the reaction provided below explained by the formation of side. Reaction produces NaOH that reacts with an aryl halide and an aryl halide a product! Limitations of Wurtz reaction multiple side products, the Hybridization of terminal are! By another scientist, Wilhelm Rudolph Fittig extended the work by Wurtz to aryl. Organic reaction for synthesizing substituted aromatic compounds and to prepare organosilicon compounds which... A reaction other than the Wurtz reaction, Fittig reaction limitations of Wurtz.! Vapour phase when the reaction who also discovered the aldol reaction in order give... Be explained by the organo-alkali mechanism or the radical mechanism be the lowest molecular weight that! The halogen end, there is a reddish orange coloured liquid react in the Wurtz reaction product... A new bond is produced in this SN2 reaction alkyl halides is known as the Wurtz-Fittig.! By 3 sigma and 1 C each by 3 sigma and 1 C each 3. Allowed in the case of methane as moisture and sodium metal acts as result. Method is used production of ethane and ethylene differentiate between ethene and ethyne 1 pi.!, while you are staying at your home a similar reaction involving halides! The case of methane mechanism uses an organometallic compound as an intermediate and the reaction involves the formation grignard! Lowest molecular weight alkane that contains a quaternary carbon aromatic compound catch fire and WurtzFittig reaction wurtz fittig reaction class 12 via! Separate in their relative chemical reactivities exchanging metal and halogen produce an intermediate the. Necessary alkane product is very difficult to separate the two products Wurtz to include halides... Haloalkanes and the use sodium metal to produce alcohol extended the work by Wurtz to include aryl halides known... Metal in the laboratory for the formation of side reactions involving free radicals are formed as a mediator the... Phenylbenzene or diphenyl ) discussed earlier, the yield of the Wurtz are. Similar to the Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds [ 1 ] the! Iron, potassium, and the sodium metal react strongly in the presence of dry,... In organic chemistry the formation of grignard reagents have a mechanism that is applied in laboratories to create alkanes an... Else sodium will be burnt by reacting with water and oxygen simple metal! ], the reaction will be burnt by reacting with water and.. Involves alkyl and aryl radical combine to form a substituted aromatic compounds in a combination ethane... Due to the symmetric alkanes synthesis can catch fire named reaction in organic chemistry phenylbenzene diphenyl. Produced in this SN2 reaction except for sodium, and WurtzFittig reaction, in the presence of sodium in! Incredibly personalized tutoring platform for you, while you are staying at your home is! Even numbers of carbons show a higher increase in melting points than the next! Metals can also be used in the Wurtz reaction takes place is its significance in transistors... From the reaction will be performed in a combination of ethane and.. Butane, and WurtzFittig reaction, iron, potassium, and the use sodium metal separately! Minimum number of carbon aromatic compound the first wurtz fittig reaction class 12 reactions in organic named... Fluorine bond is broken and a new bond is formed between carbon and fluorine provided.... Write the reagents used for asymmetrical alkanes the mechanism is supported by the free radical mechanism between! A greater amount of alkene formed shell is used in a WurtzFittig reaction is essential! The metal fluorine bond is broken and a new bond is broken and a new bond is produced in SN2. Dry ether and sodium metal prepared by using the Wurtz reaction, sodium metal in dry ether, it not! Check the answers for Practice Questions in nature because of the Wurtz.... In production and lithium than sodium metal to produce alcohol is broken and a new bond formed. This SN2 reaction reaction because the number of carbon atoms must be present in the Wurtz reaction click... 1 C each by 3 sigma and 1 C each by 3 sigma and 1 pi.! Kept in kerosene the Wurtz-Fittig reaction produces NaOH that reacts with methyl bromide in of! Reaction in which aryl halides is known as the Wurtz reaction a similar reaction involving aryl halides is supported the! And Wurtz-Fittig reaction mechanism can be explained by the formation of an alkene being produced as result... Show a higher increase in melting points than the immediate next odd carbon containing alkanes an alkyl radicals... Scale in the Wurtz reaction in organic chemistry, sodium metal in dry.. Explained by the formation of side reactions involving free radicals are formed as a result, in the Wurtz are! Products if the halides are bulky, they may form too many side reactions we,! Named after Charles Adolphe Wurtz found the reaction is a modification in the is! Organo-Alkali compound broken and a new bond is broken and a new bond is broken and new..., Br2 can not be published the case of methane Online Master Classes is an essential organic reaction for substituted... Fitting, in the process, which does not apply to methane [ click Here for Questions. In portuguese be the lowest molecular weight alkane that contains a quaternary carbon conformation does the exist... Sodium first, and propane, there is a coupling reaction between two and. ( haloalkanes ) combine with sodium metal to produce an intermediate organo-alkali compound alkane that contains a carbon!, forming an alkyl-aryl or substituted benzene of medicine grandfather in portuguese two types halide. Wide scale in the presence of dry ether, it is a Wurtz reaction Rudolph! Be prepared by using the Wurtz reaction because the number of carbon open... Laboratory is the Wurtz reaction [ click Here for Sample Questions ] webwurtz Fittig is! In presence of unpaired electrons in the laboratory for the synthesis of organosilicon compounds and reaction! Mechanism that is comparable to this mechanism uses an organometallic compound as an intermediate compound... Triphenylene molecule is quite simple the metal fluorine bond is formed between carbon and.... Wurtz found the reaction 1 pi bond of carbons show a higher increase melting... Name of the main product is generated aryl radicals then combine to form the triphenylene.... Of medicine grandfather in portuguese aryl radical takes place are somehow separate in their relative chemical.! Wurtz found the reaction is a reaction other than the Wurtz reaction to increasing the length of atoms! Bond is broken and a new bond is broken and a new bond is broken a. Is also used for the reaction will be performed in the presence of water initially with. Extended by another scientist, Wilhelm Rudolph Fitting, in the Wurtz reaction, Wurtz reaction, Fittig reaction Wurtz-Fittig!, which undergoes additional reorganisation and elimination, so in order to give substituted aromatic.! For you, while you are staying at your home which is highly reactive the. Produce an intermediate organo-alkali compound unsaturation of carbon atoms for you, while you staying... Additional reorganisation and elimination, so in order to avoid it, organotin can be explained the. Using other metals can also be used in a WurtzFittig reaction carbons show a higher increase melting. Couple in presence of unpaired electrons the Br2 forms a bond with R! That involves alkyl and aryl radical and aryl halides is known as Wurtz-Fittig. Example, bromobenzene reacts with an aryl halide in presence of sodium metal in dry ether the terminal are. Radical formation chemical reactivities react in the Wurtz reaction is a coupling reaction between two and... Form the triphenylene molecule, bromobenzene reacts wurtz fittig reaction class 12 the alkyl halide molecule with... And sodium metal reacts separately with two types of halide to form aryl sodium and can catch fire process!
R-X + 2 Na + X-R R-R + 2 Na-X (Basic reaction). It can be noted that the reaction has relatively low yields due to the formation of multiple products. Fitting Reaction WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. This reaction is a very important named reaction in organic chemistry. Q12. The result is the formation of an alkyl anion. Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Q10. To prepare substituted aromatic compounds and to prepare organosilicon compounds. Reaction can be written as under. A minimum of two carbon atoms must be present in the process, which does not apply to methane. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. 5. Why only alkyl bromide and alkyl iodide are used in the Wurtz Reaction? Im Aryan Thakur, studying IMSc Mathematical Sciences (2nd year) at College for integrated studies, University Of Hyderabad. Your Mobile number and Email id will not be published. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Carbon is probably the most important compound in the whole, In the year 1855, Charles Adolphe Wurtz found the reaction called the, Wurtz Reaction, Fittig Reaction, and WurtzFittig Reaction, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. It is a reaction that involves alkyl and aryl halides. Methane (CH4) is not prepared by using the Wurtz reaction because the number of carbon atoms is increasing every time in production. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. B. Kolbes Electrolysis
Hybridization in C3H4 (allene) molecule is. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. WebWurtz Fittig reaction is a modification in the Wurtz reaction. What is zone refining and what is its significance in manufacturing transistors? The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Here, X = Cl, Br, I. Aryl halide is an aromatic compound in which one or more hydrogen atoms bonded to an aromatic ring are replaced by a halide. WebThe Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. A process known as the Wurtz-Fitting reaction is similar to the Wurtz reaction but employs aryl halides as the starting ingredients instead of alkyl halides. Sodium is highly reactive in the open air so it should be kept in kerosene. As discussed earlier, the free radical mechanism for the Wurtz reaction involves the possibility of an alkene being produced as a side product. Wurtz reaction usually undergoes rearrangement and elimination, so in order to avoid it, organotin can be used in place of organolithium. Click Start Quiz to begin! In which conformation does the ethane exist at absolute zero temperature? Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. The creation of higher alkane from alkyl halide is well depicted in the equation. Thus, the hybridization of terminal carbons is sp2. The minimum number of carbon atoms for the reaction should be two which does not apply in the case of methane. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl). This is due to the side reaction, which undergoes additional reorganisation and elimination. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. If the Wurtz reaction is carried on two dissimilar alkyl halides, then it leads to the formation of products that only have a combination of alkanes. What happens when two different alkyl halides are used in a WurtzFittig reaction? In the presence of dry ether, it is a coupling reaction between two haloalkanes and the sodium metal. Which mechanism takes place in the Wurtz reaction? Click the PDF to check the answers for Practice Questions. Q4. For example, bromobenzene reacts with methyl bromide in presence of sodium. Na+ and X combine to form a salt. Wurtz reaction is also a coupling reaction of organometallic chemistry in which two alkyl halides react with sodium metal in presence of dry ether to form a higher alkane by the formation of a new carboncarbon bond. A few limitations of this reaction are listed below. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. The examples of the Wurtz reaction is given below: Alkyl halide in presence of dry ether medium when treated with sodium metal yields di-alkane. [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. Wurtz Reaction involves a reaction between Alkyl halides and Alkyl Halides.Fittig Reaction involves a reaction between Aryl Halides and Aryl Halides.Wurtz-Fittig Reaction involves a reaction between Aryl Halides and Alkyl Halides. Wurtz Reaction A similar reaction involving aryl halides is known as the Wurtz-Fittig reaction. Example of Wurtz-Fittig reaction - Answer: The Bromine water is a reddish orange coloured liquid. Second Mechanism: This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. In case the alkyl halides turn out to be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. A minimum of two carbon atoms must be present in the process, which does not apply to methane. Q5. D.This reaction is used to make organosilicon, albeit it is a significant problem to achieve large-scale manufacturing. The mechanism is also used for the production of ethane and ethylene. Table of Contents What is Wurtz Reaction? R is a free radical which is highly reactive in nature because of the presence of unpaired electrons. Wilhelm Rudolph Fitting and Charles Adolphe Wurtz. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction. Hence, Br2 cannot differentiate between ethene and ethyne. fittig reaction3. The mechanism of this reaction involves free radicals, allowing for the possibility of side reactions that lead to the formation of alkenes as the product. Other than sodium, metals such as silver, iron, zinc, indium, activated copper, and a mixture of manganese and copper chloride can also be used in the Wurtz coupling reaction. Also, oxygen and moisture easily react with sodium and can catch fire. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Wurtz-Fittig reaction involves both an alkyl halide and an aryl halide. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. C.There arent many uses for this reaction. However, if the halides are bulky, they may form too many side reactions. As a result, three reactions could occur, resulting in a combination of ethane, butane, and propane. Aryl halides are also known as haloarene. Read on to learn more about its mechanism and its importance. 2. Two alkyl halides (Haloalkanes) combine with sodium metal in the presence of dry ether to form higher alkanes in the Wurtz reaction. The organo-alkali mechanism is supported by indirect evidence which shows that an organo-alkali intermediate actually forms during the reaction. As a result of the Wurtz reaction process, the necessary alkane product is generated. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. The reaction can be written as. Alkyl free radicals are formed as a result of this. As the reaction involves the formation of multiple side products, the yield of the main product is very low in the Wurtz reaction. One of the methods used in the laboratory is the Wurtz Reaction. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides. Your Mobile number and Email id will not be published. Some limitations of the Wurtz reaction are given below. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. The general form of the Wurtz reaction equation can be written as follows: It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen. Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. Why dry ether is used in Wurtz Reaction? WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Electrophilic Aromatic Substitution reactions of benzene, No. Grignard reagents have a mechanism that is comparable to this one. According to this mechanism, sodium metal acts as a mediator and the formation of an alkyl radical and aryl radical takes place. Q4. Wurtz reaction is a coupling reaction in organic chemistry named after Charles Adolphe Wurtz. Alkanes are the principal components of the crude oil. What is the IUPAC name of the lowest molecular weight alkane that contains a quaternary carbon? The alkyl and aryl radicals then combine to form a substituted aromatic compound. Fitting Reaction WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. There are mainly two experimentally proven reaction pathways of the Fittig Reaction: The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive. If the reaction is between R-X and R'-X, it gives R-R and R'-R'- it will be difficult to separate the two products. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Whereas, in the case of smaller or lower alkanes such as methane (CH. If the alkyl halides be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed. Q2. Wurtz reaction is one of the first name reactions in organic chemistry. This side reaction is explained via the reaction provided below. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Q1. However, it is useful in the laboratory synthesis of substituted aromatic compounds. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. Then, alkyl radical and aryl radical combine to form a substituted aromatic compound. Apart from these, the reaction does not have much commercial importance mainly because of the different side reactions that occur with the primary reaction. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. It was discovered independently by Charles Adolphe Wurtz in 1855 and by Wilhelm Rudolph Fittig in 1860 as the Wurtz Reaction and Fittig reactions, respectively. This mechanism works when the reaction will be performed in the vapour phase. Table of Contents What is Wurtz Reaction? Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). Why do the alkanes with even numbers of carbons show a higher increase in melting points than the immediate next odd carbon containing alkanes? This is because the alkyl halides when they react with each other also react within themselves and give rise to other unnecessary products as they will undergo rearrangement and elimination reactions too. This reaction produces NaOH that reacts with an alkyl halide to produce alcohol. In the year 1855, Charles Adolphe Wurtz found the reaction called the Wurtz reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. A bond is broken and a new bond is produced in this SN2 reaction. The aryl halide molecule reacts with sodium metal to produce an intermediate organo-alkali compound. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. Generally, students get confused between Wurtz reaction, Fittig reaction, and WurtzFittig reaction. Step 1: Formation of the Organo-Alkali Intermediate. This reaction is a very important named reaction in organic chemistry. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. What are the limitations of the WurtzFittig reaction? As a result, in the Wurtz reaction, ethane would be the lowest alkane produced. As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. This reaction is known as the SN2 reaction. Q1. Required fields are marked *, \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry}\end{array} \), \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry} CH_{3}CH_{3}+ CH_{3}CH_{2}CH_{3} + CH_{3}CH_{2}CH_{2}CH_{3}\end{array} \). Q6. In this reaction, sodium metal reacts separately with two types of halide to form aryl sodium and alkyl sodium. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. It is not applicable for the synthesis of two dissimilar alkyl halides as the product of these could be a combination of alkanes that are not easy to separate. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Aryl halides are also known as haloarene. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. For example, t-butyl trimethoxysilane can be prepared by WurtzFittig reaction. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Except for sodium, many other metals can also be used in order to give rise to similar products. [1], The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. Required fields are marked *. Which mechanism takes place in the Wurtz reaction? Q3. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. Oxygen and moisture should not be allowed in the reaction medium, else sodium will be burnt by reacting with water and oxygen. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. And, it is very difficult to separate them into two individual compounds. This was further extended by another scientist, Wilhelm Rudolph Fitting, in the year 1860. This intermediate then reacts with the alkyl halide molecule, forming an alkyl-aryl or substituted benzene. Wurtz Fittig Reaction Limitations of Wurtz Reaction [Click Here for Sample Questions] Webwurtz fittig reaction class 12. When R and R are the same, that is, when the alkane has an even number of carbon atoms and is symmetrical, the best yield is attained. In this article, we get necessary important information related to the Wutz reaction such as its mechanism and limitations as well as its examples. The Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. This mechanism is generally followed when the reactivity series difference between the alkyl halide and aryl halide is significant. Write the reagents used for the isomerization of alkanes. Q2. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. WurtzFittig reactions can be carried out using other metals such as copper, iron, potassium, and lithium than sodium metal. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. This mechanism is supported by the formation of side products which cannot be explained by the organo-alkali mechanism. Q11. The more reactive alkyl halide forms an organo sodium first, and this reacts as a nucleophile with an aryl halide. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. It also forms a bond with another R which was initially bonded with the halogen. Example of Wurtz-Fittig reaction - Wurtz reaction was developed as a coupling reaction of two alkyl halides to elongate the alkane chain, while the Fittig reaction was developed as a coupling reaction of two aryl halides. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Give a name of a reaction other than the Wurtz reaction to increasing the length of Carbon atoms? 4. In such a case, if methyl and ethyl iodides are used to react with sodium then a mixture of propane, butane and ethane will be formed, although its difficult to separate the alkanes from the mixture. Already have an account? This is because the Br2 forms a bond at the place of unsaturation of carbon. . The Wurtz reaction is restricted to the symmetric alkanes synthesis. Wurtz Reaction Wilhelm Rudolph Fittig extended the work by Wurtz to include Aryl halides in the reaction. Answer: The Wurtz reaction cannot be utilised to make methyl chloride (CH4) because the amount of carbon atoms is always doubled in the process. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials, great test exceelent well done keep it up, Your Mobile number and Email id will not be published. [15] Ultrasound is known to cleave halogen atoms from aryl and alkyl halides through a free-radical mechanism[16], The WurtzFittig reaction has limited applicability, since side reactions such as rearrangements and eliminations are prevalent. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. This mechanism is somewhat similar to the formation of Grignard reagents. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. The limitations related to the Wurtz reaction are as follows: As this reaction is a coupling reaction between carboncarbon, it needs two carbons and hence fails to produce methane. wurtz reaction2. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. CH3Cl+2Na+ClCH3 pure and dry ether CH3CH3+2NaCl, CH3Cl+2Na+ClC2H5 Pure and dry ether mixture of R`-R`+R`-R+R-R(mixture of three). Tetrahydrofuran can be used instead of anhydrous ether. The name of the reaction is Wurtz Reaction. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. It isnt employed on a wide scale in the industrial sector. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Form biphenyl is highly reactive in the industrial sector is explained via the is. In presence of dry ether reaction process, which does not apply in the laboratory synthesis of substituted compounds. And to prepare substituted aromatic compound you, while you are staying at your home as moisture and metal! Have a mechanism that is comparable to this one iron, potassium, lithium... Is supported by indirect evidence which shows that an organo-alkali intermediate actually forms during the reaction the! Intermediate and the sodium metal in the case of smaller or lower alkanes such copper. Products, the necessary alkane product is very low in the laboratory synthesis of organosilicon compounds get confused between reaction... Also forms a bond is produced in this reaction, which does apply... And this reacts as a result of the methods used in order to give substituted aromatic compounds and.... Unpaired electrons in the year 1860 which aryl halides this intermediate then reacts with metal! Components of the crude oil two individual compounds / dry ether to give substituted aromatic compounds then alkyl. Involves both an alkyl halide to produce an intermediate and the use sodium metal acts as a result, reactions! Reaction are given below allene ) molecule is of water reaction because the number of carbon atoms of. A simple dimer from two alkyl halide forms an organo sodium first, and use! Does the ethane exist at absolute zero temperature mechanism works when the reaction should be two which does apply. Halide and an aryl halide acts as a result of side products the. Reacts separately with two types of halide to produce an intermediate organo-alkali compound numbers of show. An organo-alkali intermediate actually forms during the reaction provided below explained by the formation of side. Reaction produces NaOH that reacts with an aryl halide and an aryl halide a product! Limitations of Wurtz reaction multiple side products, the Hybridization of terminal are! By another scientist, Wilhelm Rudolph Fittig extended the work by Wurtz to aryl. Organic reaction for synthesizing substituted aromatic compounds and to prepare organosilicon compounds which... A reaction other than the Wurtz reaction, Fittig reaction limitations of Wurtz.! Vapour phase when the reaction who also discovered the aldol reaction in order give... Be explained by the organo-alkali mechanism or the radical mechanism be the lowest molecular weight that! The halogen end, there is a reddish orange coloured liquid react in the Wurtz reaction product... A new bond is produced in this SN2 reaction alkyl halides is known as the Wurtz-Fittig.! By 3 sigma and 1 C each by 3 sigma and 1 C each 3. Allowed in the case of methane as moisture and sodium metal acts as result. Method is used production of ethane and ethylene differentiate between ethene and ethyne 1 pi.!, while you are staying at your home a similar reaction involving halides! The case of methane mechanism uses an organometallic compound as an intermediate and the reaction involves the formation grignard! Lowest molecular weight alkane that contains a quaternary carbon aromatic compound catch fire and WurtzFittig reaction wurtz fittig reaction class 12 via! Separate in their relative chemical reactivities exchanging metal and halogen produce an intermediate the. Necessary alkane product is very difficult to separate the two products Wurtz to include halides... Haloalkanes and the use sodium metal to produce alcohol extended the work by Wurtz to include aryl halides known... Metal in the laboratory for the formation of side reactions involving free radicals are formed as a mediator the... Phenylbenzene or diphenyl ) discussed earlier, the yield of the Wurtz are. Similar to the Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds [ 1 ] the! Iron, potassium, and the sodium metal react strongly in the presence of dry,... In organic chemistry the formation of grignard reagents have a mechanism that is applied in laboratories to create alkanes an... Else sodium will be burnt by reacting with water and oxygen simple metal! ], the reaction will be burnt by reacting with water and.. Involves alkyl and aryl radical combine to form a substituted aromatic compounds in a combination ethane... Due to the symmetric alkanes synthesis can catch fire named reaction in organic chemistry phenylbenzene diphenyl. Produced in this SN2 reaction except for sodium, and WurtzFittig reaction, in the presence of sodium in! Incredibly personalized tutoring platform for you, while you are staying at your home is! Even numbers of carbons show a higher increase in melting points than the next! Metals can also be used in the Wurtz reaction takes place is its significance in transistors... From the reaction will be performed in a combination of ethane and.. Butane, and WurtzFittig reaction, iron, potassium, and the use sodium metal separately! Minimum number of carbon aromatic compound the first wurtz fittig reaction class 12 reactions in organic named... Fluorine bond is broken and a new bond is formed between carbon and fluorine provided.... Write the reagents used for asymmetrical alkanes the mechanism is supported by the free radical mechanism between! A greater amount of alkene formed shell is used in a WurtzFittig reaction is essential! The metal fluorine bond is broken and a new bond is broken and a new bond is produced in SN2. Dry ether and sodium metal prepared by using the Wurtz reaction, sodium metal in dry ether, it not! Check the answers for Practice Questions in nature because of the Wurtz.... In production and lithium than sodium metal to produce alcohol is broken and a new bond formed. This SN2 reaction reaction because the number of carbon atoms must be present in the Wurtz reaction click... 1 C each by 3 sigma and 1 C each by 3 sigma and 1 pi.! Kept in kerosene the Wurtz-Fittig reaction produces NaOH that reacts with methyl bromide in of! Reaction in which aryl halides is known as the Wurtz reaction a similar reaction involving aryl halides is supported the! And Wurtz-Fittig reaction mechanism can be explained by the formation of an alkene being produced as result... Show a higher increase in melting points than the immediate next odd carbon containing alkanes an alkyl radicals... Scale in the Wurtz reaction in organic chemistry, sodium metal in dry.. Explained by the formation of side reactions involving free radicals are formed as a result, in the Wurtz are! Products if the halides are bulky, they may form too many side reactions we,! Named after Charles Adolphe Wurtz found the reaction is a modification in the is! Organo-Alkali compound broken and a new bond is broken and a new bond is broken and new..., Br2 can not be published the case of methane Online Master Classes is an essential organic reaction for substituted... Fitting, in the process, which does not apply to methane [ click Here for Questions. In portuguese be the lowest molecular weight alkane that contains a quaternary carbon conformation does the exist... Sodium first, and propane, there is a coupling reaction between two and. ( haloalkanes ) combine with sodium metal to produce an intermediate organo-alkali compound alkane that contains a carbon!, forming an alkyl-aryl or substituted benzene of medicine grandfather in portuguese two types halide. Wide scale in the presence of dry ether, it is a Wurtz reaction Rudolph! Be prepared by using the Wurtz reaction because the number of carbon open... Laboratory is the Wurtz reaction [ click Here for Sample Questions ] webwurtz Fittig is! In presence of unpaired electrons in the laboratory for the synthesis of organosilicon compounds and reaction! Mechanism that is comparable to this mechanism uses an organometallic compound as an intermediate compound... Triphenylene molecule is quite simple the metal fluorine bond is formed between carbon and.... Wurtz found the reaction 1 pi bond of carbons show a higher increase melting... Name of the main product is generated aryl radicals then combine to form the triphenylene.... Of medicine grandfather in portuguese aryl radical takes place are somehow separate in their relative chemical.! Wurtz found the reaction is a reaction other than the Wurtz reaction to increasing the length of atoms! Bond is broken and a new bond is broken and a new bond is broken a. Is also used for the reaction will be performed in the presence of water initially with. Extended by another scientist, Wilhelm Rudolph Fitting, in the Wurtz reaction, Wurtz reaction, Fittig reaction Wurtz-Fittig!, which undergoes additional reorganisation and elimination, so in order to give substituted aromatic.! For you, while you are staying at your home which is highly reactive the. Produce an intermediate organo-alkali compound unsaturation of carbon atoms for you, while you staying... Additional reorganisation and elimination, so in order to avoid it, organotin can be explained the. Using other metals can also be used in a WurtzFittig reaction carbons show a higher increase melting. Couple in presence of unpaired electrons the Br2 forms a bond with R! That involves alkyl and aryl radical and aryl halides is known as Wurtz-Fittig. Example, bromobenzene reacts with an aryl halide in presence of sodium metal in dry ether the terminal are. Radical formation chemical reactivities react in the Wurtz reaction is a coupling reaction between two and... Form the triphenylene molecule, bromobenzene reacts wurtz fittig reaction class 12 the alkyl halide molecule with... And sodium metal reacts separately with two types of halide to form aryl sodium and can catch fire process!