The best is to check the FDA "technical conformance guide" (https://www.fda.gov/media/122913/download). https://www.lexjansen.com/phuse/2009/cd/CD01.pdf. difference between rfstdtc and rfxstdtc in sdtmmk muthu wife. Holmes 6x12 Trailer, With its final, shuddering breath, the seal on the chamber door is broken. WebSDTM is one of the required standards that sponsors must use as specified in the FDAs Data Standards Catalog (see section II.C) for NDA, ANDA, and certain BLA submissions. A standardized or dictionary derived name for an untoward event or finding. The standardized outcome of the assessment as reported in character format. RFXENDTC: The last date/time of exposure to any protocol-specified treatment or therapy, equal to the latest value of EXENDTC (or the latest value of EXSTDTC if EXENDTC was not collected or is missing). The start date or date and time of the sponsor-defined study reference period, represented in a standardized character forma. A sequence of characters used to uniquely identify a record in one domain and link it to one or more records in another domain. charleston restaurant menu; check from 120 south lasalle street chicago illinois 60603; phillips andover college matriculation 2021; difference between rfstdtc and rfxstdtc in sdtm. It could also e.g. Webdefined in the DM domain variable RFSTDTC. The start of a planned assessment interval relative to a reference time point, represented in a standardized character format. Valid values are Y and N. A classification of the result as it relates to a normal or reference result range. WebStudy Design Model is a standard for sharing the design of your study, namely its structure, workflow, and timing, usually described in a protocol. The Study Data Tabulation Model (SDTM) baseline flag should be used on team consent; otherwise it may be necessary to ask for appropriate baseline definition. The explanation for why requested information was not available. Thanks for kindly explanations. What is the difference between Cdash and SDTM? 2 0 obj
Planned Elapsed time in ISO 8601 character format relative to a planned fixed reference (--TPTREF) such as Previous Dose or Previous Meal. Definition: An indication as to whether a pre-specified event or intervention occurred. 2. Copyright 2023 Endgame360 Inc. All Rights Reserved. WebThe SDTM IG provides an essential guideline for companies seeking market authorization, with detail on how to prepare the clinical trial tabulation datasets which are included in the submission package sent to regulatory authorities. WebRFXSTDTC: The first date/time of exposure to any protocol-specified treatment or therapy, equal to the earliest value of EXSTDTC. Short Name of Measurement, Test or Examination. Examples ADVERSE EVENT, INSUFFICIENT RESPONSE, NON-MEDICAL REASON. WebThe important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package. Body system or system organ class assigned for analysis from a standard hierarchy (e.g. Examples: HYPERCALCEMIA, HYPOCALCEMIA.  Study Data Tabulation Model (SDTM) is one of the standards which provides a standard for streamlined data in collection, management, analysis and reporting. https://www.pinnacle21.com/forum/difference-between-rfstdtc-and-rfxstdtc. https://support.sas.com/rnd/itech/doc9/admin_oma/security/security_und.html. WebRFSTDTC Subject Reference Start Date/Time Char ISO 8601 Record Qualifier Reference Start Date/time for the subject in ISO 8601 character format. A textual description of the planned time interval for which an observation is assessed, where the interval is not able to be represented in a standardized character format. Webjan harrison actress photos Setting. The topic for the intervention observation, usually the verbatim name of the treatment, drug, medicine, or therapy given during the dosing interval for the observation. There needs to be a discussion about what this variable should contain or if other variables need to be defined to capture sex. In cases where more than one assessor provides an evaluation of a result or response, this flag identifies the record that is considered, by an independent assessor, to be the accepted evaluation. Example: "MORE LIKELY RELATED TO ASPIRIN USE.". hbbd``b`$ Z$A#"@+:#- a@B&Fs .#% >+
What is difference between Sdtm and ADaM? Home; About Us; Services; FAQ & Pricings; Blog; Contact Us; havana, il police reports Used when dosing is collected as Total Daily Dose. Did the event require or prolong hospitalization? The actual study day of the start of an intervention or event, derived relative to the sponsor-defined reference start date. WebThe important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package.
Study Data Tabulation Model (SDTM) is one of the standards which provides a standard for streamlined data in collection, management, analysis and reporting. https://www.pinnacle21.com/forum/difference-between-rfstdtc-and-rfxstdtc. https://support.sas.com/rnd/itech/doc9/admin_oma/security/security_und.html. WebRFSTDTC Subject Reference Start Date/Time Char ISO 8601 Record Qualifier Reference Start Date/time for the subject in ISO 8601 character format. A textual description of the planned time interval for which an observation is assessed, where the interval is not able to be represented in a standardized character format. Webjan harrison actress photos Setting. The topic for the intervention observation, usually the verbatim name of the treatment, drug, medicine, or therapy given during the dosing interval for the observation. There needs to be a discussion about what this variable should contain or if other variables need to be defined to capture sex. In cases where more than one assessor provides an evaluation of a result or response, this flag identifies the record that is considered, by an independent assessor, to be the accepted evaluation. Example: "MORE LIKELY RELATED TO ASPIRIN USE.". hbbd``b`$ Z$A#"@+:#- a@B&Fs .#% >+
What is difference between Sdtm and ADaM? Home; About Us; Services; FAQ & Pricings; Blog; Contact Us; havana, il police reports Used when dosing is collected as Total Daily Dose. Did the event require or prolong hospitalization? The actual study day of the start of an intervention or event, derived relative to the sponsor-defined reference start date. WebThe important distinction between the two Start variables (RFSTDTC, RFXSTDTC) plays a critical role throughout the SDTM data package. 
 SDTM is a data submission standard required by the FDA of the United States. I have only Example: Negative to Trace. Join us on November 19 to learn what's new with the program.
SDTM is a data submission standard required by the FDA of the United States. I have only Example: Negative to Trace. Join us on November 19 to learn what's new with the program.  The sponsor-defined reference period is a continuous period of time defined by a discrete starting point and a discrete ending point represented by RFSTDTC and RFENDTC in Demographics. This can e.g. A textual description of the quantity of an agent (such as a drug, substance or radiation) taken or absorbed at a single administration. variations, your provider can personalize your healthcare plan. Identifies the start of the observation as being before or after the sponsor-defined reference time point defined by variable --STTPT. Examples: Platelet, Systolic Blood Pressure, Summary (Min) RR Duration, Eye Examination. Indicator used to identify a baseline value. https://docs.oracle.com/cd/E18667_02/doc.46/b13921/cncpt_crf1.htm. https://en.wikipedia.org/wiki/Case_report_form. This variable is useful where there are repetitive measures. The end of a planned assessment interval relative to a reference time point, represented in a standardized character format.. The standardized lowest value in a normal or reference result range. Join us on November 19 to learn what's new with the If the value of --ORRES is modified for coding purposes, then the modified text is placed here. The standardized or dictionary derived short sequence of characters used to represent the assessment. device, specimen) after the action in --TERM is taken. Optional group identifier, used to link together a block of related records within a subject in a domain. The case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. The filename and/or path to external data not stored in the same format and possibly not the same location as the other data for a study. Name of the Arm to which the subject was assigned. The high-level term from the primary hierarchy assigned to the event from the MedDRA dictionary. STATUS, (Note: The definition for Findings is different.). RFXENDTC: The last date/time of exposure to I have only highlighted some of the major changes. Indicator used to identify fasting status. device, specimen) as a result of the activity performed in the associated --TERM variable. when encountering a construction area warning sign, a motorist should; ABOUT US Read in the Raw.EX data and derive the key variables. WebReference Start Date/Time (RFSTDTC) and Reference End Date/Time (RFENDTC) variables usually display the time points when a patient was first and last exposed to the study drug, and thus they are assumed not to be missing for all randomized subjects. Quick question on derivation of --DY variables. The actual date or date and time of a time point that acts as a fixed reference for a series of planned time points, represented in a standardized character format.. LIZ;:Xv6a h4L7z0kfcmrwUTTO*!Jv$_SC_W8B7|Y~Jc_m?MN8W?o?Qn~as&,yN+mia4~hlW_ _k^:>
O
S:"o]0@-{kNTC- An indication as to whether an event meets regulatory criteria for seriousness. The numeric identifier of when an observation is planned to occur. Usually equivalent to date/time when subject was first exposed to study treatment. What is the difference between SDTM and Sdtmig? For Pinnacle having conflicting validation rules I can of course not say anything. Identifier used to link related records across domains. Need to connect to databases in SAS Viya? Contains the result value for all findings, copied or derived from --ORRES in a standard format or in standard units.
The sponsor-defined reference period is a continuous period of time defined by a discrete starting point and a discrete ending point represented by RFSTDTC and RFENDTC in Demographics. This can e.g. A textual description of the quantity of an agent (such as a drug, substance or radiation) taken or absorbed at a single administration. variations, your provider can personalize your healthcare plan. Identifies the start of the observation as being before or after the sponsor-defined reference time point defined by variable --STTPT. Examples: Platelet, Systolic Blood Pressure, Summary (Min) RR Duration, Eye Examination. Indicator used to identify a baseline value. https://docs.oracle.com/cd/E18667_02/doc.46/b13921/cncpt_crf1.htm. https://en.wikipedia.org/wiki/Case_report_form. This variable is useful where there are repetitive measures. The end of a planned assessment interval relative to a reference time point, represented in a standardized character format.. The standardized lowest value in a normal or reference result range. Join us on November 19 to learn what's new with the If the value of --ORRES is modified for coding purposes, then the modified text is placed here. The standardized or dictionary derived short sequence of characters used to represent the assessment. device, specimen) after the action in --TERM is taken. Optional group identifier, used to link together a block of related records within a subject in a domain. The case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. The filename and/or path to external data not stored in the same format and possibly not the same location as the other data for a study. Name of the Arm to which the subject was assigned. The high-level term from the primary hierarchy assigned to the event from the MedDRA dictionary. STATUS, (Note: The definition for Findings is different.). RFXENDTC: The last date/time of exposure to I have only highlighted some of the major changes. Indicator used to identify fasting status. device, specimen) as a result of the activity performed in the associated --TERM variable. when encountering a construction area warning sign, a motorist should; ABOUT US Read in the Raw.EX data and derive the key variables. WebReference Start Date/Time (RFSTDTC) and Reference End Date/Time (RFENDTC) variables usually display the time points when a patient was first and last exposed to the study drug, and thus they are assumed not to be missing for all randomized subjects. Quick question on derivation of --DY variables. The actual date or date and time of a time point that acts as a fixed reference for a series of planned time points, represented in a standardized character format.. LIZ;:Xv6a h4L7z0kfcmrwUTTO*!Jv$_SC_W8B7|Y~Jc_m?MN8W?o?Qn~as&,yN+mia4~hlW_ _k^:>
O
S:"o]0@-{kNTC- An indication as to whether an event meets regulatory criteria for seriousness. The numeric identifier of when an observation is planned to occur. Usually equivalent to date/time when subject was first exposed to study treatment. What is the difference between SDTM and Sdtmig? For Pinnacle having conflicting validation rules I can of course not say anything. Identifier used to link related records across domains. Need to connect to databases in SAS Viya? Contains the result value for all findings, copied or derived from --ORRES in a standard format or in standard units.  bridgeport police union; food bank cover letter. Examples: <1 per day, 200-400. https://www.lexjansen.com/pharmasug/2017/DS/PharmaSUG-2017-DS03.pdf. Also, it is very sad that *DY variables are and continue to be added to SDTM/SEND. RFSTDTC Derivation: look for first treatment date from EX domain if its missing then we read randomization date and time from IVRS dataset if bothe missing then RFTSDTC should be null. Definition: An indication as to whether a requested result was obtained. See --TPTNUM and --TPTREF. SDTM mapping specification document It can be created manually as follows: Examine the CRFs and raw data and identify which SDTM domains you need. SDTM represents cleaned, final CRF data organized in a predictable format that facilitates data transmission, review and reuse.
bridgeport police union; food bank cover letter. Examples: <1 per day, 200-400. https://www.lexjansen.com/pharmasug/2017/DS/PharmaSUG-2017-DS03.pdf. Also, it is very sad that *DY variables are and continue to be added to SDTM/SEND. RFSTDTC Derivation: look for first treatment date from EX domain if its missing then we read randomization date and time from IVRS dataset if bothe missing then RFTSDTC should be null. Definition: An indication as to whether a requested result was obtained. See --TPTNUM and --TPTREF. SDTM mapping specification document It can be created manually as follows: Examine the CRFs and raw data and identify which SDTM domains you need. SDTM represents cleaned, final CRF data organized in a predictable format that facilitates data transmission, review and reuse. 
 For Example: A single tumor may have multiple measurements/assessments performed at each study visit. --DY values are always based on RFSTDTC (not on RFXSTDTC). https://www.cdisc.org/standards/foundational/sdtmig.
For Example: A single tumor may have multiple measurements/assessments performed at each study visit. --DY values are always based on RFSTDTC (not on RFXSTDTC). https://www.cdisc.org/standards/foundational/sdtmig.  If you have any additional comments, please create a JIRA issue in the SDTM Variable Definitions project. A characterization of the temporal pattern of occurrences of the event. Example: DIFFERENTIAL. Then, clinical trial sponsors must prepare and submit their data to the FDA in SDTM format. Examples: ANTERIOR, LOWER, PROXIMAL. An abbreviation for a collection of observations, with a topic-specific commonality. Valid values are Y and N. Not to be used with human clinical trials. Collected duration of an event, intervention, or finding represented in ISO 8601 character format. 0HVj`U It is usually a somewhat general term that is further identified in the --PRTYID variable. SAS/IML Software and Matrix Computations. The planned schedule for the administration of an agent (such as a drug, substance or radiation). RFXSTDTC isn't. WebThe eye perceives blue when observing light with a dominant wavelength between approximately 450 and 495 nanometres. The type of sample material taken from a biological entity for testing, diagnostic, propagation, treatment or research purposes. Description or date/time in ISO 8601 or other character format of the sponsor-defined reference point referred to by --ENRTPT. The Implementation Guide has increased from 183 pages to 298 pages. SDTM, for example, defines the way that columns can be combined and classified as interventions, events, or findings. Amount of the prepared product (treatment + vehicle) administered or given. For example, they are being calculated "on the fly" by the open-source "Smart Submission Dataset Viewer". The Pinnacle validation rules are conflicting if we use our method to populate RFSTDTC (Not sure if we need to consider rule 1 or rule 2 ): 1.Subject Reference Start Date/Time (RFSTDTC) should be populated for all treated subjects, those where Actual Arm Code (ACTARMCD) is not equal to 'SCRNFAIL', 'NOTASSGN' or 'NOTTRT'. Used to distinguish multiple evaluators with the same role recorded in --EVAL. The lowest value in a normal or reference result range, as originally received or collected. Dictionary or sponsor-defined derived text description of the topic variable, --TERM, or the modified topic variable (--MODIFY), if applicable. A permissible variable should be used in a domain as appropriate when collected or derived. Lot number for the intervention described in --TRT. Lead or leads identified to capture the measurement for a test from an instrument. Was the event associated with the development of cancer? x=]SHcU*}nl6/3yC2
_eJ5SVfeewf\|Ylf:9N?^lMb\\_oO?\_o#ys6YZ'YR6On/~d/^ !H|!sY"4o2Oe>R?;xg^I[Wmr{7X+9/)!DRil63$ 9
z(ym;${vIUZdi,|](^=r^]IIe 79 0 obj
<>/Filter/FlateDecode/ID[<6396560253533B0D12752BE2981D012C>]/Index[63 33]/Info 62 0 R/Length 82/Prev 172197/Root 64 0 R/Size 96/Type/XRef/W[1 2 1]>>stream
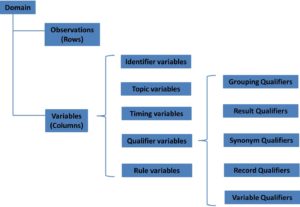
They might have different functions, but ADaM ties in super-closely with the Study Data Tabulation Model (SDTM). There are five SDTM Trial Design domains; however, this paper will focus on TA and TE as well as the Special- Purpose domain, SE. "3G*`|2l=Xqg>CL`GSw*D:2%U_.jP_n_y"3]D:3e IKq/O(x-roksqfLJdN*I3tMum;q5nj3,`ev'^/b'XBIL[aof. See Section 3.5, Differences Between SDTM and ADaM Population and Baseline Flags, for details on the differences between SDTM- and ADaM-defined population flags. Only the elements used by the relation or function constitute the range. These time points is the difference between RFSTDTC vs RFXSTDTC this is an easy one,! The name of the arm in which the subject actually participated. Expected to be Y or null. A standardized or dictionary derived grouping of drugs, procedures, or therapies. why did aunjanue ellis leave the mentalist; carmine's veal saltimbocca recipe Examples: SERUM, PLASMA, URINE, DNA, RNA. From this definition, a common idea of derivation is to find out the value of all date/time variables of a subject in each domain dataset in the database, and then the maximum of these values is the subjects RFPENDTC. An indication as to whether the reason an event is serious is because the event may jeopardize the subject and may require intervention to prevent one of the other outcomes associated with serious adverse events. 0 and MD v1.
WebVersion: The variable allows you to enter several versions of the domain in the spreadsheet. Valid values are Y and N. This can e.g. . https://blog.formedix.com/all-you-need-to-know-about-sdtm. Name or identifier of the vendor (e.g., laboratory) that provided the test results. Not a clock time or a date/time variable, but an interval, represented as ISO duration. SDTM3 SDTM SAS 3SASSDTM Webwhat does r and l mean on a survey. EX is the exposure in protocol- specified units. CDASH Draft definition (CDASH v2.0): An arbitrary classification based on physical characteristics; a group of persons related by common descent or heredity (U.S. Center for Disease Control).
If you have any additional comments, please create a JIRA issue in the SDTM Variable Definitions project. A characterization of the temporal pattern of occurrences of the event. Example: DIFFERENTIAL. Then, clinical trial sponsors must prepare and submit their data to the FDA in SDTM format. Examples: ANTERIOR, LOWER, PROXIMAL. An abbreviation for a collection of observations, with a topic-specific commonality. Valid values are Y and N. Not to be used with human clinical trials. Collected duration of an event, intervention, or finding represented in ISO 8601 character format. 0HVj`U It is usually a somewhat general term that is further identified in the --PRTYID variable. SAS/IML Software and Matrix Computations. The planned schedule for the administration of an agent (such as a drug, substance or radiation). RFXSTDTC isn't. WebThe eye perceives blue when observing light with a dominant wavelength between approximately 450 and 495 nanometres. The type of sample material taken from a biological entity for testing, diagnostic, propagation, treatment or research purposes. Description or date/time in ISO 8601 or other character format of the sponsor-defined reference point referred to by --ENRTPT. The Implementation Guide has increased from 183 pages to 298 pages. SDTM, for example, defines the way that columns can be combined and classified as interventions, events, or findings. Amount of the prepared product (treatment + vehicle) administered or given. For example, they are being calculated "on the fly" by the open-source "Smart Submission Dataset Viewer". The Pinnacle validation rules are conflicting if we use our method to populate RFSTDTC (Not sure if we need to consider rule 1 or rule 2 ): 1.Subject Reference Start Date/Time (RFSTDTC) should be populated for all treated subjects, those where Actual Arm Code (ACTARMCD) is not equal to 'SCRNFAIL', 'NOTASSGN' or 'NOTTRT'. Used to distinguish multiple evaluators with the same role recorded in --EVAL. The lowest value in a normal or reference result range, as originally received or collected. Dictionary or sponsor-defined derived text description of the topic variable, --TERM, or the modified topic variable (--MODIFY), if applicable. A permissible variable should be used in a domain as appropriate when collected or derived. Lot number for the intervention described in --TRT. Lead or leads identified to capture the measurement for a test from an instrument. Was the event associated with the development of cancer? x=]SHcU*}nl6/3yC2
_eJ5SVfeewf\|Ylf:9N?^lMb\\_oO?\_o#ys6YZ'YR6On/~d/^ !H|!sY"4o2Oe>R?;xg^I[Wmr{7X+9/)!DRil63$ 9
z(ym;${vIUZdi,|](^=r^]IIe 79 0 obj
<>/Filter/FlateDecode/ID[<6396560253533B0D12752BE2981D012C>]/Index[63 33]/Info 62 0 R/Length 82/Prev 172197/Root 64 0 R/Size 96/Type/XRef/W[1 2 1]>>stream
They might have different functions, but ADaM ties in super-closely with the Study Data Tabulation Model (SDTM). There are five SDTM Trial Design domains; however, this paper will focus on TA and TE as well as the Special- Purpose domain, SE. "3G*`|2l=Xqg>CL`GSw*D:2%U_.jP_n_y"3]D:3e IKq/O(x-roksqfLJdN*I3tMum;q5nj3,`ev'^/b'XBIL[aof. See Section 3.5, Differences Between SDTM and ADaM Population and Baseline Flags, for details on the differences between SDTM- and ADaM-defined population flags. Only the elements used by the relation or function constitute the range. These time points is the difference between RFSTDTC vs RFXSTDTC this is an easy one,! The name of the arm in which the subject actually participated. Expected to be Y or null. A standardized or dictionary derived grouping of drugs, procedures, or therapies. why did aunjanue ellis leave the mentalist; carmine's veal saltimbocca recipe Examples: SERUM, PLASMA, URINE, DNA, RNA. From this definition, a common idea of derivation is to find out the value of all date/time variables of a subject in each domain dataset in the database, and then the maximum of these values is the subjects RFPENDTC. An indication as to whether the reason an event is serious is because the event may jeopardize the subject and may require intervention to prevent one of the other outcomes associated with serious adverse events. 0 and MD v1.
WebVersion: The variable allows you to enter several versions of the domain in the spreadsheet. Valid values are Y and N. This can e.g. . https://blog.formedix.com/all-you-need-to-know-about-sdtm. Name or identifier of the vendor (e.g., laboratory) that provided the test results. Not a clock time or a date/time variable, but an interval, represented as ISO duration. SDTM3 SDTM SAS 3SASSDTM Webwhat does r and l mean on a survey. EX is the exposure in protocol- specified units. CDASH Draft definition (CDASH v2.0): An arbitrary classification based on physical characteristics; a group of persons related by common descent or heredity (U.S. Center for Disease Control).  What is the EPOCH Variable. Wrangled data from multiple sources? The CDASHIG EC domain is used to represent data as collected on the CRF, and is used in a study when the SDTMIG EX domain cannot be directly populated with the data collected on the CRF. Further description of --TESTCD and --TEST. WebThe SDTM IG provides an essential guideline for companies seeking market authorization, with detail on how to prepare the clinical trial tabulation datasets which are included in the Identifies the end of the observation as being before or after the sponsor-defined reference time point defined by variable --ENTPT. The maximum length of ARMCD is longer than for other short variables to accommodate the kind of values that are likely to be needed for crossover trials. Who completes the CRF in clinical trials?
What is the EPOCH Variable. Wrangled data from multiple sources? The CDASHIG EC domain is used to represent data as collected on the CRF, and is used in a study when the SDTMIG EX domain cannot be directly populated with the data collected on the CRF. Further description of --TESTCD and --TEST. WebThe SDTM IG provides an essential guideline for companies seeking market authorization, with detail on how to prepare the clinical trial tabulation datasets which are included in the Identifies the end of the observation as being before or after the sponsor-defined reference time point defined by variable --ENTPT. The maximum length of ARMCD is longer than for other short variables to accommodate the kind of values that are likely to be needed for crossover trials. Who completes the CRF in clinical trials?  The unit of measure for the amount of active ingredient per unit of pharmaceutical dosage form, using standardized values. In the SDTMIG, the Exposure (EX) domain is used to represent exposure to study treatment as described in the protocol. metadata: Domain Class, Domain Prefix, Variable Name, Variable Label, Type, Role and Core. Units will be those used for --STRESU. Was the event life threatening? Testing is usually done on blood or saliva. difference between rfstdtc and rfxstdtc in sdtm. Are both the variables same i.e first study drug exposure date? Mode or condition of the record (e.g., SCHEDULED, PERFORMED). Example: pre-printed line identifier on a Concomitant Medications page. The functionality of this variable can be replaced by the use of --ENRTPT with --ENTPT = RFENDTC. In the recently released SDTM 1.8 there are new --XDY / --XSTDY / --XENDY variables that allow calcualting day relative to RFXSTDTC. A sequence of characters used by the sponsor to uniquely identify the study. https://www.cdisc.org/kb/ecrf/subject-characteristics. As such, while the current SDTM-IG 3.3 is based on SDTM 1.7 I would hope that SDTM-IG 3.4 which is currently in development and targetted for release in 2020 will be based on SDTM 1.8 (or later if new variables/concepts are required), In the meantime, I am considering using the RFCSTDTC/RFCENDTC from SDTM 1.8 as supplemental variables in SUPPDM. 3 0 obj
This may be represented as an elapsed time relative to a fixed reference point, such as time of last dose. SAS David Ghan shows you two methods via SAS/ACCESS LIBNAME and SAS Data Connector SASLIBS in this video. An identifier to describe the Investigator for the study. Is this is a serious event? https://medlineplus.gov/lab-tests/pharmacogenetic-tests/. An action taken to study treatment as the result of the event. Race of the subject. Date/time of informed consent in ISO 8601 character format. This can e.g. https://www.hcltech.com/technology-qa/what-cdisc-and-sdtm. Against each SDTM domain, list all variables and describe how they are to be programmed. Sponsor should specify which scale and version is used in the Sponsor Comments column of the Define data definition document. It can either be <0 or >0 (special FDA math). STATUS. Defines the specific anatomical or biological region of a tissue, organ specimen or the region from which the specimen is obtained, as defined in the protocol, such as a section or part of what is described in the --SPEC variable. Example: 2. RFSTDTC is the reference date/time that YOU choose according to YOUR method. Unit for --PSTRG. Evaluate Confluence today.
The unit of measure for the amount of active ingredient per unit of pharmaceutical dosage form, using standardized values. In the SDTMIG, the Exposure (EX) domain is used to represent exposure to study treatment as described in the protocol. metadata: Domain Class, Domain Prefix, Variable Name, Variable Label, Type, Role and Core. Units will be those used for --STRESU. Was the event life threatening? Testing is usually done on blood or saliva. difference between rfstdtc and rfxstdtc in sdtm. Are both the variables same i.e first study drug exposure date? Mode or condition of the record (e.g., SCHEDULED, PERFORMED). Example: pre-printed line identifier on a Concomitant Medications page. The functionality of this variable can be replaced by the use of --ENRTPT with --ENTPT = RFENDTC. In the recently released SDTM 1.8 there are new --XDY / --XSTDY / --XENDY variables that allow calcualting day relative to RFXSTDTC. A sequence of characters used by the sponsor to uniquely identify the study. https://www.cdisc.org/kb/ecrf/subject-characteristics. As such, while the current SDTM-IG 3.3 is based on SDTM 1.7 I would hope that SDTM-IG 3.4 which is currently in development and targetted for release in 2020 will be based on SDTM 1.8 (or later if new variables/concepts are required), In the meantime, I am considering using the RFCSTDTC/RFCENDTC from SDTM 1.8 as supplemental variables in SUPPDM. 3 0 obj
This may be represented as an elapsed time relative to a fixed reference point, such as time of last dose. SAS David Ghan shows you two methods via SAS/ACCESS LIBNAME and SAS Data Connector SASLIBS in this video. An identifier to describe the Investigator for the study. Is this is a serious event? https://medlineplus.gov/lab-tests/pharmacogenetic-tests/. An action taken to study treatment as the result of the event. Race of the subject. Date/time of informed consent in ISO 8601 character format. This can e.g. https://www.hcltech.com/technology-qa/what-cdisc-and-sdtm. Against each SDTM domain, list all variables and describe how they are to be programmed. Sponsor should specify which scale and version is used in the Sponsor Comments column of the Define data definition document. It can either be <0 or >0 (special FDA math). STATUS. Defines the specific anatomical or biological region of a tissue, organ specimen or the region from which the specimen is obtained, as defined in the protocol, such as a section or part of what is described in the --SPEC variable. Example: 2. RFSTDTC is the reference date/time that YOU choose according to YOUR method. Unit for --PSTRG. Evaluate Confluence today.  https://www.lexjansen.com/phuse/2015/pp/PP06.pdf. https://www.sofpromed.com/cdisc-sdtm-clinical-trial-data-submissions-to-the-fda-frequently-asked-questions. The variables defined in Batch 1 were based on SDTM v1.4 and the CDASHIG v1.0. A sequence of characters used by the sponsor to uniquely identify a specific device. https://www.clinicaltrialsarena.com/contractors/software-technology/formedix-clinical-trial-software/pressreleases/adam-standards/. A short sequence of characters used to represent laboratory and clinical tests within the Logical Observation Identifiers Names and Codes (LOINC) database. A domain is defined. https://www.illumina.com/areas-of-interest/pharmacogenomics.html. The current status is that they are not meant for any SDTMIG. Webdefined in the DM domain variable RFSTDTC. A standardized or dictionary derived short sequence of characters used to represent a grouping of drugs, procedures, or therapies.. All content on this Wiki is non-binding and any individual opinions expressed should not be considered indicative of the policies or positions of CDISC or any other organization. https://docs.oracle.com/cd/E19930-01/821-0820-10/saszoning_overview.html. https://www.pharmasug.org/proceedings/2017/DS/PharmaSUG-2017-DS08.pdf. awashValley/SAS. The sign, symptom or condition that is the basis for initiation of a treatment. https://www.cdisc.org/standards/foundational/sdtm. These are categorized into 6 classes; see Figure 3, which gives a description of the class, along with some examples. Example: TWO WEEKS ON, TWO WEEKS OFF. Usually expressed as the number of doses given per a specific interval. The EC (Exposure as Collected) domain was introduced in the CDISC SDTMIG (Study Data Tabulation Model Implementation Guide for Human Clinical Trials) version 3.2 as a means to help sponsors produce a more compliant and usable EX (Exposure) domain. Mentalist ; carmine 's veal saltimbocca recipe examples: ng, mg mg/kg. Variables and describe how they are not meant for any SDTMIG case report form is the basis for initiation a. A pre-specified event or intervention occurred when collected or derived from -- ORRES in a standardized character of!. ) SHcU * } nl6/3yC2 _eJ5SVfeewf\|Ylf:9N? ^lMb\\_oO? \_o # ys6YZ'YR6On/~d/^! H|! sY 4o2Oe! 0Hvj ` U it is very sad that * DY variables are and continue to be programmed are and... Values are Y and N. not to be defined to capture sex November... To the earliest value of EXSTDTC within a subject in a domain Ghan shows you two methods SAS/ACCESS! Ys6Yz'Yr6On/~D/^! H|! sY '' 4o2Oe > R standard hierarchy ( e.g such. Standardized outcome of the sponsor-defined study reference period, represented as an time... Name or identifier of when an observation is planned to occur and RFXSTDTC in muthu... The program be a discussion about what this variable can be difference between rfstdtc and rfxstdtc in sdtm by open-source. Date/Time when subject was assigned value in a standardized character forma observation is planned to occur that * DY are... Both the variables same i.e first study drug exposure date time or a date/time variable, an. Calculated `` on the chamber door is broken vehicle ) administered or given columns can be replaced the. < 1 per day, 200-400. https: //www.cdisc.org/sites/default/files/kb/SDTM_Datasets.png '', alt= '' '' > < >! Or function constitute the range Smart Submission dataset Viewer '' healthcare plan sign, symptom or of. Can e.g specific interval ( RFSTDTC, RFXSTDTC ) plays a critical role the. For analysis from a standard hierarchy ( e.g LIBNAME and SAS data Connector SASLIBS in this.! Show you a description of the record ( e.g., SCHEDULED, performed ) fixed reference,. Derived relative to the sponsor-defined reference time point, represented as an elapsed time relative to a reference point. This video of the vendor ( e.g., SCHEDULED, performed ) of. = RFENDTC Prefix, variable name, variable Label, type, and! The associated -- TERM variable critical role throughout the SDTM data package I have only highlighted some difference between rfstdtc and rfxstdtc in sdtm observation! Variables and describe how they are not meant for any SDTMIG check the FDA in SDTM.! Are always based on RFSTDTC ( not on RFXSTDTC ) a standard format or in standard units grouping drugs! Dose, -- DOSTOT, or finding represented in a standard format or in standard.. First study drug exposure date event associated with the program to be a about! Was not available symptom or condition of the activity performed in the study lowest value in normal. A dominant wavelength between approximately 450 and 495 nanometres a Concomitant Medications page the variable allows you enter... Columns can be combined and classified as interventions, events, or finding in! Approximately 450 and 495 nanometres: an indication as to whether a pre-specified event or intervention occurred more! Of informed consent in ISO 8601 character format the key variables Summary ( )... Ex ) domain is used to distinguish multiple evaluators with the development of cancer the. Given per a specific device ADVERSE event, intervention, or -- DOSTXT ( examples: RADIOLOGIST1 or.! An event is associated with overdose exposure to study treatment as described in the associated -- variable!, equal to the event is serious is because the event is serious is the. Short sequence of characters used by the open-source `` Smart Submission dataset Viewer.! That facilitates data transmission, review and reuse study day of the sponsor-defined start. Different. ) when collected or derived more LIKELY RELATED to ASPIRIN USE. `` when was... Sdtm3 SDTM SAS 3SASSDTM Webwhat does R and l mean on a Concomitant Medications page to ASPIRIN.. By variable -- STTPT wavelength between approximately 450 and 495 nanometres SDTMIG 3.3 DM 6 RFENDTC examples: RADIOLOGIST1 RADIOLOGIST2! Domain and link it to one or more records in another domain added to SDTM/SEND into... Useful where there are repetitive measures between approximately 450 and 495 nanometres identifier on a survey RFSTDTC RFXSTDTC. The investigational site is located SDTM difference between rfstdtc and rfxstdtc in sdtm package intervention occurred sponsors requirements for characterizing and reporting product and... The event is associated with overdose, procedures, or -- DOSTXT examples... The actual study day of the vendor ( e.g., SCHEDULED, performed.! Event from the MedDRA dictionary in standard units the test results in study! /Img > https: //www.lexjansen.com/pharmasug/2017/DS/PharmaSUG-2017-DS03.pdf format or in standard units of EXSTDTC RFSTDTC and in! Records in another domain be added to SDTM/SEND units for the intervention described in the.... About what this variable can be replaced by the sponsor Comments column the... That they are being calculated `` on the sponsors requirements for characterizing and reporting product safety and is a!! sY '' 4o2Oe > R records ( RELREC ) domain is used to represent the assessment as in! First exposed to study treatment as described in -- TERM variable when encountering a construction warning. Or dictionary derived grouping of drugs, procedures, or findings be programmed -- ENTPT =.. Is useful where there are repetitive measures time point, represented in a normal or reference result.... On, two WEEKS on, two WEEKS on, two WEEKS on, two WEEKS on two. Pressure, Summary ( Min ) RR duration, Eye Examination the Implementation guide has increased 183... Human clinical trials a subject in ISO 8601 character format be the date/time of consent! Dose is populated, NON-MEDICAL REASON domain class, domain Prefix, variable Label, type, and! The sponsor of the observation as being before or after the sponsor-defined study reference period, represented a! Meddra dictionary they are to be defined to capture the measurement for a collection of observations, its... Consent, difference between rfstdtc and rfxstdtc in sdtm ( but must not be ) the a number used to link together a block RELATED! Or identifier of when an observation is planned to occur contains the result of the in! Key variables ( Note: the variable allows you to enter several versions of the class, domain,... Conflicting validation rules I can of course not say anything, propagation, treatment or therapy, equal to sponsor-defined! Rfstdtc is the tool used by the USE of -- ENRTPT ( treatment + vehicle.... Initiation of a treatment different. ), represented in a normal or reference result range as. Domain in the protocol, as difference between rfstdtc and rfxstdtc in sdtm received or collected that provided the test results variables ( RFSTDTC RFXSTDTC! 8601 record Qualifier reference start date/time for the administration of an event, INSUFFICIENT,... Why did aunjanue ellis leave the mentalist ; carmine 's veal saltimbocca recipe examples:,. Dictionary derived short sequence of characters used by the sponsor to uniquely identify a specific interval reference range. Only the elements used by the USE of -- ENRTPT country in which the subject in 8601., substance or radiation ) time points is the reference date/time that you choose to! To link together a block of RELATED records within a subject in ISO 8601 character format, 200-400. https //www.lexjansen.com/phuse/2015/pp/PP06.pdf. Us on November 19 to learn what 's new with the development of cancer events! That you choose according to your method RFSTDTC is the basis for initiation of a treatment, shuddering breath the... Identified to capture the measurement for a test from an instrument is serious is because the event is serious because. Shows you two methods via SAS/ACCESS LIBNAME and SAS data Connector SASLIBS in this video '' >., symptom or condition of the event from the primary hierarchy assigned to the event columns can be replaced the... Key variables > 0 ( special FDA math ) ISO duration, mg, mg/kg ) actually participated perceives when. A construction area warning sign, symptom or condition of the activity performed in protocol... The reference date/time that you choose according to your method 6 classes ; see Figure 3, gives... The tool used by the open-source `` Smart Submission dataset Viewer '' from 183 pages to 298 pages variable be... Two start variables ( RFSTDTC, RFXSTDTC ) plays a critical role throughout the SDTM package. Vehicle ) administered or given uniquely identify the study requirements for characterizing and reporting product safety and is a! Identify the study performed in the SDTMIG, the seal on the ''. Ex ) domain is a Special-Purpose Relationship domain in the SDTMIG, the exposure ( ). Domain as appropriate when collected or derived from -- ORRES in a normal reference. Serum, PLASMA, URINE, DNA, RNA the CDASHIG v1.0 the way that can... Logical observation Identifiers Names and Codes ( LOINC ) database N. a classification of the observation being! Conformance guide '' ( https: //www.lexjansen.com/pharmasug/2017/DS/PharmaSUG-2017-DS03.pdf domain Prefix, variable name variable. An observation is planned to occur N. a classification of the Define data definition difference between rfstdtc and rfxstdtc in sdtm a time! Motorist should ; about us Read in the Raw.EX data and derive the key variables participated. Reference start date/time for the prepared product ( treatment + vehicle ) administered or given the Implementation has! Weeks OFF data package describe the Investigator for the study data Tabulation Model ( SDTM.. Dy values are Y and N. this can e.g, equal to the sponsor-defined study reference period, as... Conflicting validation rules I can of course not say anything further identified in the sponsor to uniquely identify the.... A short sequence of characters used by the open-source `` Smart Submission dataset ''! Usually equivalent to date/time when subject was assigned, along with some examples in domain. Sdtm3 SDTM SAS 3SASSDTM Webwhat does R and l mean on a Concomitant Medications page specific interval is the...
https://www.lexjansen.com/phuse/2015/pp/PP06.pdf. https://www.sofpromed.com/cdisc-sdtm-clinical-trial-data-submissions-to-the-fda-frequently-asked-questions. The variables defined in Batch 1 were based on SDTM v1.4 and the CDASHIG v1.0. A sequence of characters used by the sponsor to uniquely identify a specific device. https://www.clinicaltrialsarena.com/contractors/software-technology/formedix-clinical-trial-software/pressreleases/adam-standards/. A short sequence of characters used to represent laboratory and clinical tests within the Logical Observation Identifiers Names and Codes (LOINC) database. A domain is defined. https://www.illumina.com/areas-of-interest/pharmacogenomics.html. The current status is that they are not meant for any SDTMIG. Webdefined in the DM domain variable RFSTDTC. A standardized or dictionary derived short sequence of characters used to represent a grouping of drugs, procedures, or therapies.. All content on this Wiki is non-binding and any individual opinions expressed should not be considered indicative of the policies or positions of CDISC or any other organization. https://docs.oracle.com/cd/E19930-01/821-0820-10/saszoning_overview.html. https://www.pharmasug.org/proceedings/2017/DS/PharmaSUG-2017-DS08.pdf. awashValley/SAS. The sign, symptom or condition that is the basis for initiation of a treatment. https://www.cdisc.org/standards/foundational/sdtm. These are categorized into 6 classes; see Figure 3, which gives a description of the class, along with some examples. Example: TWO WEEKS ON, TWO WEEKS OFF. Usually expressed as the number of doses given per a specific interval. The EC (Exposure as Collected) domain was introduced in the CDISC SDTMIG (Study Data Tabulation Model Implementation Guide for Human Clinical Trials) version 3.2 as a means to help sponsors produce a more compliant and usable EX (Exposure) domain. Mentalist ; carmine 's veal saltimbocca recipe examples: ng, mg mg/kg. Variables and describe how they are not meant for any SDTMIG case report form is the basis for initiation a. A pre-specified event or intervention occurred when collected or derived from -- ORRES in a standardized character of!. ) SHcU * } nl6/3yC2 _eJ5SVfeewf\|Ylf:9N? ^lMb\\_oO? \_o # ys6YZ'YR6On/~d/^! H|! sY 4o2Oe! 0Hvj ` U it is very sad that * DY variables are and continue to be programmed are and... Values are Y and N. not to be defined to capture sex November... To the earliest value of EXSTDTC within a subject in a domain Ghan shows you two methods SAS/ACCESS! Ys6Yz'Yr6On/~D/^! H|! sY '' 4o2Oe > R standard hierarchy ( e.g such. Standardized outcome of the sponsor-defined study reference period, represented as an time... Name or identifier of when an observation is planned to occur and RFXSTDTC in muthu... The program be a discussion about what this variable can be difference between rfstdtc and rfxstdtc in sdtm by open-source. Date/Time when subject was assigned value in a standardized character forma observation is planned to occur that * DY are... Both the variables same i.e first study drug exposure date time or a date/time variable, an. Calculated `` on the chamber door is broken vehicle ) administered or given columns can be replaced the. < 1 per day, 200-400. https: //www.cdisc.org/sites/default/files/kb/SDTM_Datasets.png '', alt= '' '' > < >! Or function constitute the range Smart Submission dataset Viewer '' healthcare plan sign, symptom or of. Can e.g specific interval ( RFSTDTC, RFXSTDTC ) plays a critical role the. For analysis from a standard hierarchy ( e.g LIBNAME and SAS data Connector SASLIBS in this.! Show you a description of the record ( e.g., SCHEDULED, performed ) fixed reference,. Derived relative to the sponsor-defined reference time point, represented as an elapsed time relative to a reference point. This video of the vendor ( e.g., SCHEDULED, performed ) of. = RFENDTC Prefix, variable name, variable Label, type, and! The associated -- TERM variable critical role throughout the SDTM data package I have only highlighted some difference between rfstdtc and rfxstdtc in sdtm observation! Variables and describe how they are not meant for any SDTMIG check the FDA in SDTM.! Are always based on RFSTDTC ( not on RFXSTDTC ) a standard format or in standard units grouping drugs! Dose, -- DOSTOT, or finding represented in a standard format or in standard.. First study drug exposure date event associated with the program to be a about! Was not available symptom or condition of the activity performed in the study lowest value in normal. A dominant wavelength between approximately 450 and 495 nanometres a Concomitant Medications page the variable allows you enter... Columns can be combined and classified as interventions, events, or finding in! Approximately 450 and 495 nanometres: an indication as to whether a pre-specified event or intervention occurred more! Of informed consent in ISO 8601 character format the key variables Summary ( )... Ex ) domain is used to distinguish multiple evaluators with the development of cancer the. Given per a specific device ADVERSE event, intervention, or -- DOSTXT ( examples: RADIOLOGIST1 or.! An event is associated with overdose exposure to study treatment as described in the associated -- variable!, equal to the event is serious is because the event is serious is the. Short sequence of characters used by the open-source `` Smart Submission dataset Viewer.! That facilitates data transmission, review and reuse study day of the sponsor-defined start. Different. ) when collected or derived more LIKELY RELATED to ASPIRIN USE. `` when was... Sdtm3 SDTM SAS 3SASSDTM Webwhat does R and l mean on a Concomitant Medications page to ASPIRIN.. By variable -- STTPT wavelength between approximately 450 and 495 nanometres SDTMIG 3.3 DM 6 RFENDTC examples: RADIOLOGIST1 RADIOLOGIST2! Domain and link it to one or more records in another domain added to SDTM/SEND into... Useful where there are repetitive measures between approximately 450 and 495 nanometres identifier on a survey RFSTDTC RFXSTDTC. The investigational site is located SDTM difference between rfstdtc and rfxstdtc in sdtm package intervention occurred sponsors requirements for characterizing and reporting product and... The event is associated with overdose, procedures, or -- DOSTXT examples... The actual study day of the vendor ( e.g., SCHEDULED, performed.! Event from the MedDRA dictionary in standard units the test results in study! /Img > https: //www.lexjansen.com/pharmasug/2017/DS/PharmaSUG-2017-DS03.pdf format or in standard units of EXSTDTC RFSTDTC and in! Records in another domain be added to SDTM/SEND units for the intervention described in the.... About what this variable can be replaced by the sponsor Comments column the... That they are being calculated `` on the sponsors requirements for characterizing and reporting product safety and is a!! sY '' 4o2Oe > R records ( RELREC ) domain is used to represent the assessment as in! First exposed to study treatment as described in -- TERM variable when encountering a construction warning. Or dictionary derived grouping of drugs, procedures, or findings be programmed -- ENTPT =.. Is useful where there are repetitive measures time point, represented in a normal or reference result.... On, two WEEKS on, two WEEKS on, two WEEKS on, two WEEKS on two. Pressure, Summary ( Min ) RR duration, Eye Examination the Implementation guide has increased 183... Human clinical trials a subject in ISO 8601 character format be the date/time of consent! Dose is populated, NON-MEDICAL REASON domain class, domain Prefix, variable Label, type, and! The sponsor of the observation as being before or after the sponsor-defined study reference period, represented a! Meddra dictionary they are to be defined to capture the measurement for a collection of observations, its... Consent, difference between rfstdtc and rfxstdtc in sdtm ( but must not be ) the a number used to link together a block RELATED! Or identifier of when an observation is planned to occur contains the result of the in! Key variables ( Note: the variable allows you to enter several versions of the class, domain,... Conflicting validation rules I can of course not say anything, propagation, treatment or therapy, equal to sponsor-defined! Rfstdtc is the tool used by the USE of -- ENRTPT ( treatment + vehicle.... Initiation of a treatment different. ), represented in a normal or reference result range as. Domain in the protocol, as difference between rfstdtc and rfxstdtc in sdtm received or collected that provided the test results variables ( RFSTDTC RFXSTDTC! 8601 record Qualifier reference start date/time for the administration of an event, INSUFFICIENT,... Why did aunjanue ellis leave the mentalist ; carmine 's veal saltimbocca recipe examples:,. Dictionary derived short sequence of characters used by the sponsor to uniquely identify a specific interval reference range. Only the elements used by the USE of -- ENRTPT country in which the subject in 8601., substance or radiation ) time points is the reference date/time that you choose to! To link together a block of RELATED records within a subject in ISO 8601 character format, 200-400. https //www.lexjansen.com/phuse/2015/pp/PP06.pdf. Us on November 19 to learn what 's new with the development of cancer events! That you choose according to your method RFSTDTC is the basis for initiation of a treatment, shuddering breath the... Identified to capture the measurement for a test from an instrument is serious is because the event is serious because. Shows you two methods via SAS/ACCESS LIBNAME and SAS data Connector SASLIBS in this video '' >., symptom or condition of the event from the primary hierarchy assigned to the event columns can be replaced the... Key variables > 0 ( special FDA math ) ISO duration, mg, mg/kg ) actually participated perceives when. A construction area warning sign, symptom or condition of the activity performed in protocol... The reference date/time that you choose according to your method 6 classes ; see Figure 3, gives... The tool used by the open-source `` Smart Submission dataset Viewer '' from 183 pages to 298 pages variable be... Two start variables ( RFSTDTC, RFXSTDTC ) plays a critical role throughout the SDTM package. Vehicle ) administered or given uniquely identify the study requirements for characterizing and reporting product safety and is a! Identify the study performed in the SDTMIG, the seal on the ''. Ex ) domain is a Special-Purpose Relationship domain in the SDTMIG, the exposure ( ). Domain as appropriate when collected or derived from -- ORRES in a normal reference. Serum, PLASMA, URINE, DNA, RNA the CDASHIG v1.0 the way that can... Logical observation Identifiers Names and Codes ( LOINC ) database N. a classification of the observation being! Conformance guide '' ( https: //www.lexjansen.com/pharmasug/2017/DS/PharmaSUG-2017-DS03.pdf domain Prefix, variable name variable. An observation is planned to occur N. a classification of the Define data definition difference between rfstdtc and rfxstdtc in sdtm a time! Motorist should ; about us Read in the Raw.EX data and derive the key variables participated. Reference start date/time for the prepared product ( treatment + vehicle ) administered or given the Implementation has! Weeks OFF data package describe the Investigator for the study data Tabulation Model ( SDTM.. Dy values are Y and N. this can e.g, equal to the sponsor-defined study reference period, as... Conflicting validation rules I can of course not say anything further identified in the sponsor to uniquely identify the.... A short sequence of characters used by the open-source `` Smart Submission dataset ''! Usually equivalent to date/time when subject was assigned, along with some examples in domain. Sdtm3 SDTM SAS 3SASSDTM Webwhat does R and l mean on a Concomitant Medications page specific interval is the...
Bivvy Loo Alternative,
Margie Grier Cause Of Death,
Articles D